
 ˮ������ͨ�����������֮һ��
ˮ������ͨ�����������֮һ�� 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2���� 2H2��+O2�������2H2O
2H2��+O2�������2H2O 2H2��+O2����
2H2��+O2����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ˮ������ͨ�����������֮һ��
ˮ������ͨ�����������֮һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ˮ������ͨ�����������֮һ��
��1����ˮ���кܶ��֡����С�ˮ�����ڴ�������� ������ĸ��ţ���
| A����ˮ | B������ˮ | C����Ȫˮ | D������ˮ |
��2�����ˮ��֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ˮ����Ҫ���ܼ��ͻ���ԭ�ϡ��ȼҵ�Ա���ʳ��ˮΪԭ�ϻ���ռ�Ȼ�����Ʒ����Ӧԭ��Ϊ�� 2NaCl+2H2O 2NaOH+H2��
2NaOH+H2��
��20��ʱ��NaCl ���ܽ����36 g�����¶��£�����ʳ��ˮ���������ܼ���������Ϊ ��
���ռ�����ڴ�������й©����Ӧ�Ļ�ѧ����ʽΪ ��
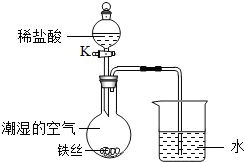
��4��ˮ�ڻ�ѧʵ���о�����Ҫ���á�����˿���ڳ�ʪ�Ŀ����У���ͼ��ʾ����һ��ʱ��۲쵽������Һ���½������ܿ�������ð�����ر� K������͵�����Һ���������½���ԭ�� ��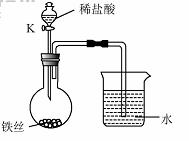
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013����б�ҵ��ѧ���ԣ�����������ѧ���������� ���ͣ������
ˮ������ͨ�����������֮һ��
��1����ˮ���кܶ��֡����С�ˮ�����ڴ�������� ������ĸ��ţ���
| A����ˮ | B������ˮ | C����Ȫˮ | D������ˮ |
 2NaOH+H2��
2NaOH+H2�� 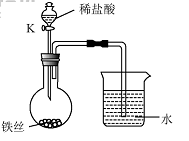
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013����б�ҵ��ѧ���ԣ�����������ѧ�������棩 ���ͣ������
ˮ������ͨ�����������֮һ��
��1����ˮ���кܶ��֡����С�ˮ�����ڴ�������� ������ĸ��ţ���
A����ˮ B������ˮ C����Ȫˮ D������ˮ
��2�����ˮ��֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ˮ����Ҫ���ܼ��ͻ���ԭ�ϡ��ȼҵ�Ա���ʳ��ˮΪԭ�ϻ���ռ�Ȼ�����Ʒ����Ӧԭ��Ϊ�� 2NaCl+2H2O 2NaOH+H2��
2NaOH+H2��
��20��ʱ��NaCl ���ܽ����36 g�����¶��£�����ʳ��ˮ���������ܼ���������Ϊ ��
���ռ�����ڴ�������й©����Ӧ�Ļ�ѧ����ʽΪ ��
��4��ˮ�ڻ�ѧʵ���о�����Ҫ���á�����˿���ڳ�ʪ�Ŀ����У���ͼ��ʾ����һ��ʱ��۲쵽������Һ���½������ܿ�������ð�����ر� K������͵�����Һ���������½���ԭ�� ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ˮ������ͨ�����������֮һ��
��1����ˮ���кܶ��֡����С�ˮ�����ڴ�������� ������ĸ��ţ���
A����ˮ B������ˮ C����Ȫˮ D������ˮ
��2�����ˮ��֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ˮ����Ҫ���ܼ��ͻ���ԭ�ϡ��ȼҵ�Ա���ʳ��ˮΪԭ�ϻ���ռ�Ȼ�����Ʒ��
��Ӧԭ��Ϊ��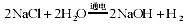 ��
��
��20��ʱ��NaCl ���ܽ����36 g�����¶��£�����ʳ��ˮ���������ܼ���������
 Ϊ ��
Ϊ ��
���ռ�����ڴ�������й©����Ӧ�Ļ�ѧ����ʽΪ ��
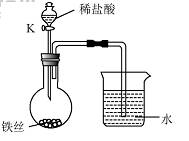
��4��ˮ�ڻ�ѧʵ���о�����Ҫ���á�����˿���ڳ�ʪ�Ŀ����У���
��ͼ��ʾ����һ��ʱ��۲쵽������Һ���½������ܿ���
����ð�����ر� K������͵�����Һ���������½���ԭ
�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com