
���� ��1���������������������������кͷ�Ӧ�������ɣ�
��2�������ؽ������ж���ԭ���жϣ����ƻ�����ĵ����ʽṹ��ʹ֮ʧȥ�������ܣ�
��� �⣺��1��θ���к������ᣬ��������������������кͷ�Ӧ����Ӧ����ʽΪ��3HCl+Al��OH��3=AlCl3+3H2O
��2���ؽ������ж���ԭ�����ƻ�����ĵ����ʽṹ���������Ҫ�ɷ��ǵ����ʣ������塢������ţ���к��е����ʣ����ü����塢������ţ�̣��ɷ�ֹ���屾���ĵ����ʱ��ƻ��������ڽⶾ��
�ʴ�Ϊ����1��HCl��3HCl+Al��OH��3=AlCl3+3H2O
��2��ţ�̣������ʣ���
���� �����ѶȲ��������������������к�θ���ԭ�����ж�ԭ������ȷ��������Ĺؼ����ڣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
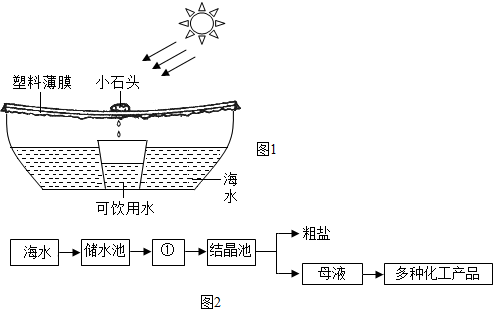
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ռ� | B�� | ��ʯ�� | C�� | ���� | D�� | ʳ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
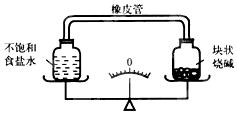
| A�� | ָ��ƫ��ʳ����Һһ����Ũ���ռ���� | |
| B�� | ָ��ƫ�ң�ʳ����Һһ�����ͣ��ռ�� | |
| C�� | ָ��ƫ��ʳ����Һһ����Ũ���ռ�� | |
| D�� | ָ��ƫ�ң�ʳ����Һһ����Ũ���ռ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1��8 | B�� | 2��1 | C�� | 4��5 | D�� | 9��10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | �� | �� | �� | �� | �� |
| ���� | ���� | ������ | ��ʯ�� | ���� | ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com