�⣺��1����ԭ������ͭ������Ϊm����������ͭ��Ӧ�����������x����������ͭ������y
CuO+H
2SO
4�TCuSO
4+H
2O
80 98 160
m x y
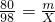
x=


y=2m
���������������ͭ��Һ��ַ�Ӧ�������ӵ�����Ϊa����ʣ��ϡ���ᷴӦ����������Ϊb��
Fe+CuSO
4�TFeSO
4+Cu ������������
56 160 64 64-56=8
2m a

Fe+H
2SO
4�TFeSO
4+H
2��
56 98
b 100g��14%-


b=8-0.7m
��Ӧǰ������������䣬�������ᷴӦ����������������������ͭ��Ӧ�������ӵ�����������a=b
0.1m=8-0.7m ���m=10g
�������֪����������ȫ��װ��Ϊ��������������������������ΪZ��
H
2SO
4 ��FeSO
4
98 152
14g��100% Z
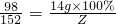
Z=

g
�������۵�����W=

g��

��100%=8g
��2�����������֪����Ӧ�����ɵĶ�����̼������Ϊ��
25g+115g+100g-231.2g=8.8g
��25gʯ��ʯ��̼��Ƶ�����Ϊp����Ӧ�����Ȼ��Ƶ�����Ϊq
CaCO
3+2HCl�TCaCl
2+H
2O+CO
2��
100 111 44
p q 8.8g

��ã�p=20g

��� q=22.2g
ʯ��ʯ��̼��Ƶ���������Ϊ
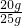
��100%=80.0%
�ڷ�Ӧ����Һ�����ʵ���������Ϊ��
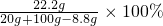
=20.0%
�𣺣�1��10��8����2����ʯ��ʯ��̼��Ƶ�����������80.0%���ڷ�Ӧ����Һ�����ʵ�����������20.0%
��������1���������⣬��CuSO
4+Fe=FeSO
4+Cu�����Կ�����ת����ͭ������һ��ʹ�����������ӵĹ��̣�����Ŀ��ȷ��˵�����˺�Ĺ���������Ͷ�������������ͬ����ֻ��˵������ͭ���ܽ��������Ȼ��ʣ�࣬ʣ�����������������ۣ����������۵�������ǰһ���������ӵ�������ȣ�������һ������ϵ����������������ͭ���������������֪����������ȫ��װ��Ϊ��������������������غ��������������۵�����W��ֵ��
��2�����������غ㶨�ɣ��ձ����������ʼ��ٵ������������ɵĶ�����̼���������ɶ�����̼������������̼��������ᷴӦ�ķ���ʽ�����̼��ơ��Ȼ��Ƶ����������������ݾͿ��Խ����йصļ��㣮
���������1����ʱ�������������������ԭ���ǽ������Ĺؼ���Ҫʹ��������ۺ͵õ��Ĺ���������ȱ���ʹ�������Ķ����Fe�����������۵��������û���ͭ�������ӵ�������ȣ�

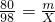 x=
x=
 y=2m
y=2m

 b=8-0.7m
b=8-0.7m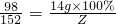 Z=
Z= g
g g��
g�� ��100%=8g
��100%=8g ��ã�p=20g
��ã�p=20g ��� q=22.2g
��� q=22.2g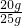 ��100%=80.0%
��100%=80.0% 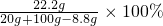 =20.0%
=20.0% 

