
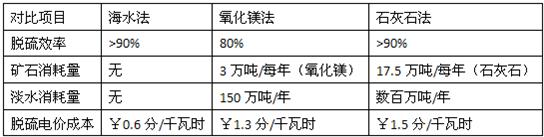
| �Ա���Ŀ | ��ˮ�� | ����þ�� | ʯ��ʯ�� |
| ������ | ��90% | 80% | ��90% |
| ��ʯ������ | �� | 3���/�� ������þ�� |
17.5���/�� ��ʯ��ʯ�� |
| ��ˮ������ | �� | 150���/�� | �������/�� |
| �����۳ɱ� | ��0.6��/�ȵ� | ��1.3��/�ȵ� | ����1.5��/�ȵ� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �Ա���Ŀ | ��ˮ�� | ����þ�� | ʯ��ʯ�� |
| ������ | ��90% | 80% | ��90% |
| ��ʯ������ | �� | 3���/�� ������þ�� |
17.5���/�� ��ʯ��ʯ�� |
| ��ˮ������ | �� | 150���/�� | �������/�� |
| �����۳ɱ� | ��0.6��/�ȵ� | ��1.3��/�ȵ� | ����1.5��/�ȵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ������з���Ƶĺ�ˮ�������գ��ѳɹ�Ӧ���ڴ����ͻ����顣�乤�մ��������ǣ��Ӹ߶��µĺ�ˮϴ�Ѵ��¶���ȼú�����е�SO2��Ȼ����������ֽӴ����ٲ�ȡ��������Ȼ��ˮ(pH��8.1��8.3)��ϵȴ�ʩ��ʹ����ָ��ӽ���Ȼ��ˮ�����ŷš�
ij�������糧(6�� 600MW)�ĺ�ˮ�������������跨�Ա���Ŀ���±���ʾ��
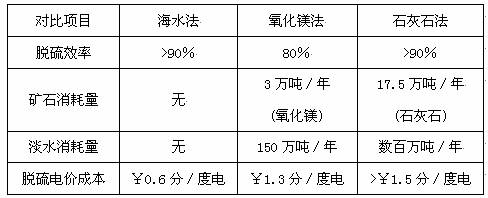
��1�������������ݣ����ɺ�ˮ����������ƺ;��ޡ�
������ �� ��
������ ��
��2����֪ϴ��������ĺ�ˮ��������Ӧ�IJ����������ƺ����ᣬ��д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007���ʮ�߽조��ԭ����ȫ������ѧ����ѧ���ʺ�ʵ��������������ɽ�����������Ծ��������棩 ���ͣ������
| �Ա���Ŀ | ��ˮ�� | ����þ�� | ʯ��ʯ�� |
| ������ | ��90% | 80% | ��90% |
| ��ʯ������ | �� | 3���/�� ������þ�� | 17.5���/�� ��ʯ��ʯ�� |
| ��ˮ������ | �� | 150���/�� | �������/�� |
| �����۳ɱ� | ��0.6��/�ȵ� | ��1.3��/�ȵ� | ����1.5��/�ȵ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com