������̽��ˮ����ɵ�ʵ�飮��ͼ�ǵ��ˮʵ���ʾ��ͼ��
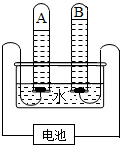
��1��д���÷�Ӧ�Ļ�ѧ����ʽ______��
��2��A�˽ӵ��______�����������������
��3�����ˮ�Ĺ����У������ı�����ǣ�д���ƣ�______��
��4����ʵ���֤��ˮ��Ԫ����ɣ�ˮ���ɣ�д���ƣ�______��ɵģ�
��5��ˮ��Ԫ�ش�����ʽ��______�������̬������̬������
��6������B�Թ�������IJ���������������______��
��7�������ˮ����ˮ3.6g����A�Թ�����������ķ��Ӹ���ԼΪ______��
��8��Ϊ�˽�һ���ⶨˮ�е�Ԫ����ɵ������ȣ�ij�Ƽ�С���ͬѧ���������ʵ�飨װ����ͼ����
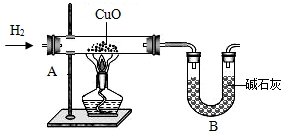
ͨ��������Ӧǰ��װ��A��B��������������m��H����m��O����1��8��������ֵƫ�ߣ���ԭ�������______�������ţ�
A��ͨ�������������������B��װ��A�ڹܿ���ˮ����
C������ͭû����ȫ��ԭD��װ��Bͬʱ�����˿����е�ˮ������CO
2��





 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д� ��ʱ�ƿ�������ϰϵ�д�
��ʱ�ƿ�������ϰϵ�д�
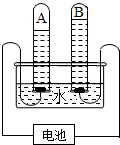
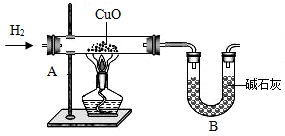
 ��2012?��������ģ��������̽��ˮ����ɵ�ʵ�飮��ͼ�ǵ��ˮʵ���ʾ��ͼ��
��2012?��������ģ��������̽��ˮ����ɵ�ʵ�飮��ͼ�ǵ��ˮʵ���ʾ��ͼ��



