
���� ���ݸ�����ת����ϵ��Ӧ�Ĺ��̷���ÿ����Ӧ�����⣬����ֱ�ӷ���ÿ�����⣬�Ӹ�������Ϣ���Ҷ�Ӧ����Ϣ��
��� �⣺
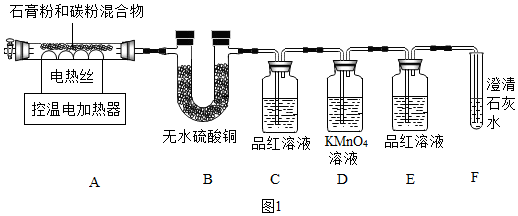
һ���ߴ�CaO���Ʊ�
ʵ������з��֣�B����ˮ����ͭ������˵��������ˮ����������Ϣ��ʾ��SO2��ʹƷ����Һ��ɫ������������֤���ж����������ɣ����Զ�Ӧ������ΪC�е�Ʒ����Һ��ɫ����װ��D�����ն����������¸��������ɫ��Eװ�õ�������֤�����������Ѿ���ȫ�����գ�������ʯ��ˮ����ǣ����Ƕ�����̼���£����Ƕ�����������֤��������̼���ɵ�������װ�� E�������Ա仯����������ĸ��ţ���װ��F�� ����ʯ��ˮ����������ж�����̼����̼���ڸ������������������ơ�ˮ��������̼�Ͷ�������Ӧ�Ļ�ѧ����ʽΪ2CaSO4•2H2O+C$\frac{\underline{\;����\;}}{\;}$4H2O��+2CaO+2SO2��+CO2��
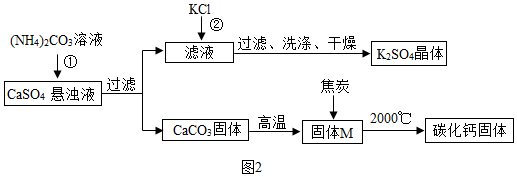
��������غ͵�ʯ���Ʊ�
��1����Ӧ����̼��狀�����Ʒ�Ӧ����̼��ƺ�����泥���Ӧ�Ļ�ѧ����ʽΪ CaSO4+��NH4��2CO3�TCaCO3��+��NH4��2 SO4������̼��������������������壬����Ҫ֤������M�в���CaCO3�ķ�����ȡ������������ϡ���ᣬ������ð����
��2����Ӧ�ڵĻ�ѧ����ʽΪ��NH4��2SO4+2KCl�TK2SO4��+2NH4Cl����������ر�ע�˳������ţ������е�֪ʶ������ؿ��ܣ�˵���ڸ����������ܽ�������С�����Ը÷�Ӧ������K2SO4�����ԭ���Ǹ��¶��£�K2SO4���ܽ��С��
��3��ʵ�������ͨ��ͨ�벻��������̼�Ŀ���������Ժ������ɵĶ�����̼�������ĸ��ţ�����̼������Ʒת��Ϊ�����ƺͶ�����̼��Ȼ�����ɵĶ�����̼�ñ�װ����ȫ���գ�Ҳ����װ�ñ����������Ӿ������ɵĶ�����̼��Ϊ��������̼��ȫ���ն�����������Թ��У������ڷ�Ӧ��ȫ��Ӧ�ü���ͨ���ȥ�˶�����̼�Ŀ������Ҳ�ļ�ʯ���DZ����Ҳ�Ŀ�������װ���ڵ��¶�����̼�ȱ����գ�Ӱ��ʵ������ȷ�ԣ�
���ԣ�
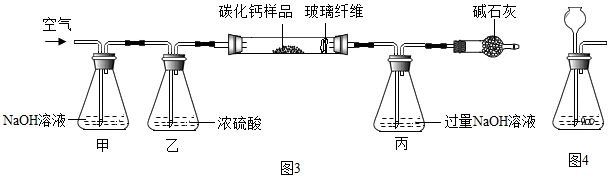
��װ�ü������� ��ȥ�����е�CO2���壮��Ӧ��ȫ���������ͨ�������Ŀ���� ʹ������װ���ڵ�CO2���屻��װ�ó�����գ�
��̼Ԫ�ص�����Ϊ4.4g��$\frac{12}{12+16��2}$��100%=1.2g����Ԫ�ص�����Ϊ6g��$\frac{40}{40+12+16��3}$��100%=2.4g������Ԫ�ص�����Ϊ3.76g-1.2g-2.4g=0.16g������Ʒ�иơ�̼������������Ϊ2.4g��1.2g��0.16g=30��15��2��
��Ӧ�������Ƶ�����Ϊ0.16g�£�$\frac{16}{40+16}$��100%��=0.56g�����������еĸ�Ԫ�ص�����Ϊ0.56g-0.16g=0.4g
��Ӧ��̼�����еĸ�Ԫ�ص�����2.4g-0.4g=2g����̼�����и�ԭ�Ӻ�̼ԭ�ӵĸ�����Ϊ$\frac{2g}{40}$��$\frac{1.2g}{12}$=1��2
����CaCx�Ļ�ѧʽCaC2��
��Ϊ���Ʒ�Ӧ���ٶ��Եõ�ƽ�ȵ��������ӷ�Ӧ��ĽǶȣ�̼���ƿ�����һ�㷴Ӧ������ˮ�ĺ�����һЩ�������¶ȵ�һЩ������ˮ�ĵμ��ٶȿɿ�Ҳ�������������Ը����ĸĽ��������е���ACD��
�ʴ�Ϊ��
һ���ߴ�CaO���Ʊ�
Ʒ����Һ��ɫ�� E������ʯ��ˮ�����
2CaSO4•2H2O+C $\frac{\underline{\;����\;}}{\;}$2CaO+2SO2��+CO2��+4H2O
��������غ͵�ʯ���Ʊ�
��1��CaSO4+��NH4��2CO3�TCaCO3��+��NH4��2 SO4��
ȡ������������ϡ���ᣬ������ð����
��2�����¶��£�K2SO4���ܽ��С��
��3���ٳ�ȥ�����е�CO2���壻 ʹ������װ���ڵ�CO2���屻��װ�ó�����գ�
��30��15��2��
�������
��Ӧ�������Ƶ�����Ϊ0.16g�£�$\frac{16}{40+16}$��100%��=0.56g�����������еĸ�Ԫ�ص�����Ϊ0.56g-0.16g=0.4g
��Ӧ��̼�����еĸ�Ԫ�ص�����2.4g-0.4g=2g����̼�����и�ԭ�Ӻ�̼ԭ�ӵĸ�����Ϊ$\frac{2g}{40}$��$\frac{1.2g}{12}$=1��2
̼���ƵĻ�ѧʽΪCaC2��
��ACD
���� ��ͼ�����л�ý����Ŀ�������Ϣ�������ڽ����Ŀʱ�ȿ�����������ʲô��Ȼ���������ȥ��������ͼ����ȥѰ�ҽ�����õ���Ϣ�������������Ϣ������Ч�ԣ���������ʵ�����븴�ӵ�ת��ͼ��ȣ���ʵ�ܼܻ��������߿���˵ת��ͼ�ṩ�����龳���������֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��ȡҺ��ҩƷ | B�� |  ����Һ��ҩƷ | C�� |  ���������� | D�� |  Ϩ��ƾ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
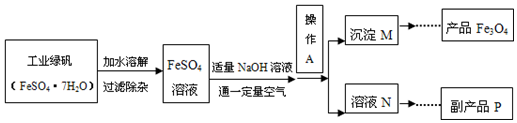
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
| �ܽ��/g | NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 |
| KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | |
| A�� | KNO3���ܽ�ȱ�NaCl�� | |
| B�� | 20��ʱ����17gNaCl ��KNO3����50gˮ�У�����ܽ⣬���ܵõ�������Һ | |
| C�� | 60��ʱ�����͵�KNO3��Һ�ȱ��͵�NaCl��Һ���������������� | |
| D�� | ���Ȼ�����Һ�еõ������Ȼ��ƾ��壬�ɲ�ȡ�����¶ȵķ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ����Һ�� | B�� |  �������� | C�� |  ��ȡҺ����� | D�� |  ϡ��Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ˮ������ÿ�춼��Ҫ�����ʣ�
ˮ������ÿ�춼��Ҫ�����ʣ��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com