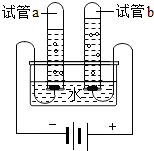
Ė®ŹĒČĖĄą×ī±¦¹óµÄ×ŌȻ׏Ō“£¬ŅĄ¾ŻĖłŃ§»ÆѧÖŖŹ¶»Ų“š£ŗ
£Ø1£©Š”Ć÷ĄūÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆĢ½¾æĖ®µÄ×é³É£¬Š“³öøĆ·“Ó¦µÄ»ÆѧŹ½±ķ“ļŹ½
£¬ĶصēŅ»¶ĪŹ±¼äŗó£¬ŹŌ¹ÜaŹÕ¼Æµ½µÄĘųĢåŹĒ
ĒāĘų
ĒāĘų
£¬Õż¼«Óėøŗ¼«²śÉśĘųĢåµÄĢå»ż±ČŹĒ
1£ŗ2
1£ŗ2
£»ĪŖĮĖ
ŌöĒæĖ®µÄµ¼µēŠŌ
ŌöĒæĖ®µÄµ¼µēŠŌ
£¬ŠčŅŖŌŚĖ®ÖŠ¼ÓČėĻ”ĮņĖį»ņĒāŃõ»ÆÄĘČÜŅŗ£®
£Ø2£©½«”°ęĻ¶šŅ»ŗÅ”±ĖĶÉĻĢ«æյĻš¼żĶĘĮ¦¾Ž“ó£¬ŅņĪŖĖüĖłŹ¹ÓƵÄČ¼ĮĻŹĒŅŗĒā”¢ÖśČ¼¼ĮŹĒŅŗŃõ£¬ŌņŅŗĒāČ¼ÉյĻÆѧŹ½±ķ“ļŹ½ĪŖ
£®
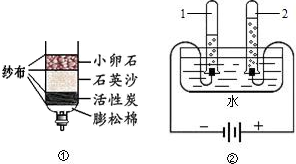
£Ø3£©ĪŖĮĖĢ½¾æ”°Ė®µÄ¾»»Æ”±¹ż³Ģ£¬Ä³ŹµŃ銔×é“Ó»¤³ĒŗÓÖŠČ”ĮĖĖ®Ńł£¬¹Ū²ģµ½Ė®Ńł»ė×Ē£¬³Ź»ĘÉ«£¬ÓŠŅģĪ¶£¬ÓŠ¹ĢĢ劔æÅĮ££®ĻÖ¶ŌĖ®Ńł½ųŠŠČēĻĀ“¦Ąķ²½Öč£ŗ
¢Ł¼ÓČė»īŠŌĢæĪüø½Ė®ÖŠµÄŃÕÉ«ŗĶŅģĪ¶£»
¢ŚĻņĖ®ŃłÖŠ¼ÓČėĆ÷·Æ½Į°čČܽā£¬¾²ÖĆŅ»¶ĪŹ±¼äŗ󣬽ųŠŠ
¹żĀĖ
¹żĀĖ
£ØĢī²Ł×÷Ćū³Ę£©£¬³żČ„ĮĖĖ®ŃłÖŠµÄ¹ĢĢ劔æÅĮ££¬Čō½ųŠŠĶźøĆ²Ł×÷ŗ󣬷¢ĻÖĀĖŅŗČŌ¾É»ė×Ē£¬Ōģ³ÉÕāŅ»ĻÖĻóµÄŌŅņæÉÄÜŹĒ
ĀĖÖ½ĘĘĖš£¬¹żĀĖŹ±ŅŗĆęøßÓŚĀĖÖ½µÄ±ßŃŲµČ
ĀĖÖ½ĘĘĖš£¬¹żĀĖŹ±ŅŗĆęøßÓŚĀĖÖ½µÄ±ßŃŲµČ
£®
£Ø4£©×ŌĄ“Ė®³§ÓƶžŃõ»ÆĀČĻū¶¾É±¾ś£¬¶žŃõ»ÆĀȵĻÆѧŹ½ĪŖ
ClO2
ClO2
£¬ĘäÖŠĀČŌŖĖŲµÄ»ÆŗĻ¼ŪĪŖ
+4
+4
£®
£Ø5£©¾®Ė®ÖŠŗ¬ÓŠ½Ļ¶ąµÄøĘ”¢Ć¾Ąė×Ó£¬ĪŖ½µµĶÓ²¶Č£¬ČÕ³£Éś»īÖŠŹ¹ÓĆÓ²Ė®»į“ųĄ“Šķ¶ąĀé·³£¬¼ŅĶ„Éś»īÖŠ³£ÓĆĄ“½µµĶĖ®Ó²¶ČµÄ·½·ØŹĒ
Öó·Š
Öó·Š
£®

 Ė®ŹĒČĖĄą×ī±¦¹óµÄ×ŌȻ׏Ō“£¬ŅĄ¾ŻĖłŃ§»ÆѧÖŖŹ¶»Ų“š£ŗ
Ė®ŹĒČĖĄą×ī±¦¹óµÄ×ŌȻ׏Ō“£¬ŅĄ¾ŻĖłŃ§»ÆѧÖŖŹ¶»Ų“š£ŗ



 Ė®ŹĒČĖĄą×ī±¦¹óµÄ×ŌȻ׏Ō“£®ÓŅĶ¼ŹĒµē½āĖ®µÄŹµŃé×°ÖĆ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
Ė®ŹĒČĖĄą×ī±¦¹óµÄ×ŌȻ׏Ō“£®ÓŅĶ¼ŹĒµē½āĖ®µÄŹµŃé×°ÖĆ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ £Ø2010?×Ō¹±£©Ė®ŹĒČĖĄą×ī±¦¹óµÄ×ŌȻ׏Ō“£®ČēĶ¼ŹĒµē½āĖ®µÄŹµŃé×°ÖĆ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
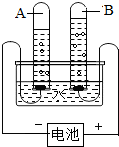
£Ø2010?×Ō¹±£©Ė®ŹĒČĖĄą×ī±¦¹óµÄ×ŌȻ׏Ō“£®ČēĶ¼ŹĒµē½āĖ®µÄŹµŃé×°ÖĆ£¬»Ų“šĻĀĮŠĪŹĢā£ŗ Ė®ŹĒČĖĄą×ī±¦¹óµÄ×ŌȻ׏Ō“£®Š”Ć÷ĄūÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆĢ½¾æĖ®µÄ×é³É£®ĶصēŅ»¶ĪŹ±¼äŗó£¬ŹŌ¹ÜAÓėŹŌ¹ÜBĖłŹÕ¼Æµ½µÄĘųĢåĢå»żÖ®±ČŌ¼ĪŖ
Ė®ŹĒČĖĄą×ī±¦¹óµÄ×ŌȻ׏Ō“£®Š”Ć÷ĄūÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆĢ½¾æĖ®µÄ×é³É£®ĶصēŅ»¶ĪŹ±¼äŗó£¬ŹŌ¹ÜAÓėŹŌ¹ÜBĖłŹÕ¼Æµ½µÄĘųĢåĢå»żÖ®±ČŌ¼ĪŖ