
”¾ĢāÄæ”æij»ÆѧŹµŃ銔×éŅŖ²ā¶ØŅ»ÖÖ“æ¼īѳʷ֊Ģ¼ĖįÄʵÄŗ¬Į棬²¢ÓĆŅ»¶ØČÜÖŹÖŹĮæ·ÖŹżµÄĻ”ĮņĖį½ųŠŠŹµŃ飬¼ĒĀ¼ŹµŃ鏿¾ŻČēĻĀ£Øѳʷ֊ŌÓÖŹ²»ŗ¬ÄĘŌŖĖŲ”¢²»ČÜÓŚĖ®Ņ²²»ÓėĮņĖį·“Ó¦£©
µŚŅ»“Ī | µŚ¶ž“Ī | µŚČż“Ī | |
¼ÓČėµÄĻ”ĮņĖįµÄÖŹĮæ(g) | 100 | 100 | 100 |
¼ÓČėµÄѳʷµÄÖŹĮæ(g) | 20 | 40 | 42 |
Éś³ÉĘųĢåµÄÖŹĮæ(g) | 2.2 | a | 4.4 |
£Ø1£©ÉĻ±ķÖŠa=_________£»
£Ø2£©øł¾ŻŅŃÖŖĢõ¼žĮŠ³öĒó½āµŚČż“Ī²Ī¼Ó·“Ó¦µÄ“æ¼īµÄÖŹĮæ(x)µÄ±ČĄżŹ½__________£»
£Ø3£©½«µŚ¶ž“ĪŹµŃéĖłµĆµÄČÜŅŗÕō·¢µō6.2gĖ®£¬ĖłµĆ²»±„ŗĶČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ___________£»
£Ø4£©ČōÅäÖĘŹµŃéÖŠĖłÓĆĻ”ĮņĖį200g£¬ŠčŅŖÓĆČÜÖŹÖŹĮæ·ÖŹżĪŖ98%µÄÅØĮņĖįµÄÖŹĮæĪŖ________£»
£Ø5£©ÕāÖÖ“æ¼īѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ______________”£
”¾“š°ø”æ 4.4£» 106£ŗ44=x£ŗ4.4g£» 14.2%£» 20g£» 26.5%”£
”¾½āĪö”æ£Ø1£©ÓɱķæÉÖŖ£¬20gµÄѳʷæÉŅŌÉś³É2.2gµÄĘųĢ壬¹Ź40gµÄѳʷ×ī¶ąæÉŅŌÉś³ÉĘųĢåµÄÖŹĮæĪŖ4.4g£¬¹Źa=4.4g£»
£Ø2£©É赌ȿ“Ī²Ī¼Ó·“Ó¦µÄ“æ¼īµÄÖŹĮæĪŖx£¬Éś³ÉĮņĖįÄʵÄÖŹĮæĪŖy
Na2CO3+ H2SO4= Na2SO4+ H2O+ CO2ӟ
106 146 44
x y 4.4g
![]() =
=![]() =
=![]() y=14.6g £¬x=10.6g
y=14.6g £¬x=10.6g
£Ø3£©µŚ¶ž“ĪÉś³ÉĘųĢåµÄÖŹĮæĪŖ4.4g£¬100gµÄĻ”ĮņĖįÓė40gµÄѳʷĒ”ŗĆĶźČ«·“Ó¦£¬Éč
ÓÉÉĻæÉÖŖ£¬x=10.6g£¬½«µŚ¶ž“ĪŹµŃéĖłµĆµÄČÜŅŗÕō·¢µō6.2gĖ®£¬ĖłµĆ²»±„ŗĶČÜŅŗµÄÖŹĮæĪŖ100g+10.6g-4.4g-6.2g=100g£¬ĖłµĆ²»±„ŗĶČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ![]() =14.6%
=14.6%
£Ø4£©ÉčĻ”ĮņĖįÖŠČÜÖŹµÄÖŹĮæĪŖm
Na2CO3+ H2SO4= Na2SO4+ H2O+ CO2ӟ
98 44
m 4.4g
![]() =
=![]() m=9.8g
m=9.8g
¹ŹøĆ Ļ”ĮņĖįČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ![]() =9.8%
=9.8%
ÅäÖĘŹµŃéÖŠĖłÓĆĻ”ĮņĖį200g£¬ŠčŅŖÓĆČÜÖŹÖŹĮæ·ÖŹżĪŖ98%µÄÅØĮņĖįµÄÖŹĮæĪŖ200g![]() 9.8%
9.8%![]() 98%=20g
98%=20g
£Ø5£©ÕāÖÖ“æ¼īѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ![]() =26.5%
=26.5%
“š£ŗ±ķÖŠa=4.4g£¬µŚČż“Ī²Ī¼Ó·“Ó¦µÄ“æ¼īµÄÖŹĮæ(x)µÄ±ČĄżŹ½![]() =
=![]() £¬ĖłµĆ²»±„ŗĶČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ14.6%£¬ŠčŅŖÓĆČÜÖŹÖŹĮæ·ÖŹżĪŖ98%µÄÅØĮņĖįµÄÖŹĮæĪŖ20g£¬ÕāÖÖ“æ¼īѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ26.5%”£
£¬ĖłµĆ²»±„ŗĶČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ14.6%£¬ŠčŅŖÓĆČÜÖŹÖŹĮæ·ÖŹżĪŖ98%µÄÅØĮņĖįµÄÖŹĮæĪŖ20g£¬ÕāÖÖ“æ¼īѳʷ֊Ģ¼ĖįÄʵÄÖŹĮæ·ÖŹżĪŖ26.5%”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æA”«H ŹĒ³õÖŠ»Æѧ֊µÄ³£¼ūĪļÖŹ”£
Ēė½įŗĻĖüĆĒÖ®¼äµÄ×Ŗ»Æ¹ŲĻµĶ¼£¬»Ų“šĻĀĮŠĪŹĢā(Ķ¼ÖŠ”°”ś”±±ķŹ¾ĪļÖŹ¼ä“ęŌŚ×Ŗ»Æ¹ŲĻµ)”£
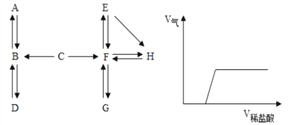
(1)A ŌŚ±ź×¼×“æöĻĀŹĒĆܶČ×īŠ”µÄĘųĢ壬A µÄ»ÆѧŹ½ŹĒ_______£»A”¢B”¢C ÖŠŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲ£¬C”śB µÄ·“Ó¦ĻÖĻóĪŖÉś³É»ĘÉ«ČÜŅŗ£¬ÓÉ“ĖĶʶĻÓėC·“Ӧɜ³ÉBµÄĪļÖŹŹĒ__________”£
(2)DĪŖĘųĢåµ„ÖŹ£¬Š“³öB”śDµÄ»Æѧ·½³ĢŹ½___________________________________________”£
(3)¹¤Ņµ³£ÓĆG”śF·“Ó¦Į¶Ģś£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ__________________________________”£
(4)E”śH ²śÉś°×É«³Įµķ£¬¹żĀĖŗóĻņĀĖŅŗÖŠµĪ¼ÓĻ”ŃĪĖį£¬²śÉśĘųĢåĢå»żÓėĖł¼ÓĻ”ŃĪĖįĢå»żµÄ¹ŲĻµČēÉĻĶ¼ĖłŹ¾”£ĀĖŅŗÖŠµÄČÜÖŹŹĒ_______£»Š“³öF”śEµÄ»Æѧ·½³ĢŹ½___________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŗŚÄ¾¶śŹĒŅ»ÖÖÓŖŃų·įø»µÄŹ³ÓĆ¾ś£¬ŗ¬ÓŠČĖĢå±ŲŠčµÄøĘ”¢Ģś”¢ŗśĀܲ·ĖŲ”¢Ī¬ÉśĖŲµČÓŖŃųĪļÖŹ£¬¾³£Ź³ÓĆæɲ¹³äČĖĢå¶ŌĢśµČĪ¢ĮæŌŖĖŲµÄŠčĒó”£ĘäÖŠÓŖŃų³É·ÖµÄ¾ßĢåŗ¬ĮæČē׏ĮĻæØʬĖłŹ¾”£Ēėøł¾Ż×ŹĮĻ»Ų“šĻĀĮŠĪŹĢā£ŗ

(1)ŗŚÄ¾¶śÖŠĖłŗ¬µÄĢśŹĒÖø___(Ģī×ÖÄøŠņŗÅ).
A. ·Ö×ÓB.Ō×ÓC.ŌŖĖŲ
(2)ĮņĖįŃĒĢś(FeSO4)ŹĒ³£¼ū²¹ĢśĄą±£½”Ę·ÖŠµÄÓŠŠ§³É·ÖÖ®Ņ»£¬Ēėøł¾ŻĘä»ÆѧŹ½¼ĘĖć£ŗ
¢ŁĮņĖįŃĒĢśµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ___£¬
¢ŚĮņĖįŃĒĢśÖŠĢś”¢Įņ”¢ŃõČżÖÖŌŖĖŲµÄÖŹĮæ±ČĪŖ___£»
¢ŪÓė200gøÉŗŚÄ¾¶śÖŠµÄĢśŌŖĖŲÖŹĮæĻąµČµÄĮņĖįŃĒĢśµÄÖŹĮæĪŖ___mg.
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ¼×ŗĶŅŅŌŚŅ»¶ØĢõ¼žĻĀ·“Ӧɜ³É±ūŗĶ¶””£½įŗĻĪ¢¹ŪŹ¾ŅāĶ¼·ÖĪö£¬ĻĀĮŠ½įĀŪÕżČ·µÄŹĒ( )
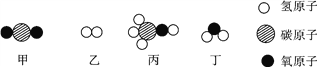
A. ±ūµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ24 B. Éś³ÉµÄ±ūŗĶ¶”µÄ·Ö×ÓøöŹż±ČĪŖ2”Ć1
C. ·“Ó¦Ē°ŗó·Ö×Ó×ÜŹż²»±ä D. ·¢Éś·“Ó¦µÄ¼×ŗĶŅŅµÄÖŹĮæ±ČĪŖ22”Ć3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻņAgNO3ČÜŅŗÖŠ¼ÓČėŅ»¶ØÖŹĮæAlŗĶFeµÄ»ģŗĻ·ŪÄ©£¬³ä·Ö·“Ó¦ŗó¹żĀĖ£¬µĆµ½ĀĖŌüŗĶĒ³ĀĢÉ«ĀĖŅŗ![]() ¹ŲÓŚøĆĀĖŌüŗĶĀĖŅŗÓŠĻĀĮŠĖÄÖÖĖµ·Ø£ŗ
¹ŲÓŚøĆĀĖŌüŗĶĀĖŅŗÓŠĻĀĮŠĖÄÖÖĖµ·Ø£ŗ
![]() ĻņĀĖŌüÖŠ¼ÓČėĻ”ŃĪĖį£¬Ņ»¶ØÓŠĘųÅŻ²śÉś£®
ĻņĀĖŌüÖŠ¼ÓČėĻ”ŃĪĖį£¬Ņ»¶ØÓŠĘųÅŻ²śÉś£®
![]() ĻņĀĖŅŗÖŠ¼ÓČėĻ”ŃĪĖį£¬Ņ»¶ØÓŠ³Įµķ²śÉś£®
ĻņĀĖŅŗÖŠ¼ÓČėĻ”ŃĪĖį£¬Ņ»¶ØÓŠ³Įµķ²śÉś£®
![]() ĀĖŌüÖŠŅ»¶ØƻӊAl£¬æÉÄÜŗ¬ÓŠAg£®
ĀĖŌüÖŠŅ»¶ØƻӊAl£¬æÉÄÜŗ¬ÓŠAg£®
![]() ĀĖŅŗÖŠŅ»¶Øŗ¬ÓŠFe(NO3)2£¬æÉÄÜŗ¬ÓŠAl(NO3)3£®
ĀĖŅŗÖŠŅ»¶Øŗ¬ÓŠFe(NO3)2£¬æÉÄÜŗ¬ÓŠAl(NO3)3£®
ŅŌÉĻĖµ·ØÖŠÕżČ·µÄøöŹżŹĒ
A. 0øö B. 1øö C. 2øö D. 3øö
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĶ¼ŹĒŹµŃéŹŅÖĘČ”ĘųĢå³£ÓƵÄŅĒĘ÷»ņ×°ÖĆ”£

(1)Ķ¼ÖŠŅĒĘ÷BµÄĆū³ĘŹĒ___£»
(2)ČōÓĆĀČĖį¼ŲÖĘČ”ŃõĘų£¬³żÉĻŹöŅĒĘ÷Ķā»¹ŠčŅŖŌö¼ÓŅ»ÖÖŅĒĘ÷£¬ĘäĆū³ĘŹĒ___£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_____________£»ČōŃ”ÓĆC×°ÖĆŹÕ¼ÆŃõĘų£¬æŖŹ¼ŹÕ¼ÆµÄŹŹŅĖŹ±æĢŹĒ___£»
(3)ČōÓĆG×°ÖĆÖĘČ”¶žŃõ»ÆĢ¼ĘųĢåµÄÓŵćŹĒ____________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĖłŹ¾µÄĖÄøöĶ¼Ļń£¬ÄÜÕżČ··“Ó³¶ŌÓ¦±ä»Æ¹ŲĻµµÄŹĒ
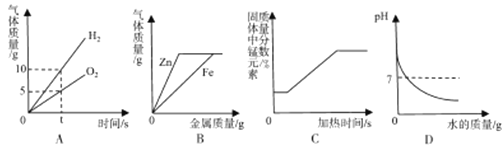
A£®µē½āĖ®
B£®ĻņĮ½·ŻĶźČ«ĻąĶ¬µÄĻ”ŃĪĖįÖŠ·Ö±š¼ÓČėŠæ·ŪŗĶĢś·Ū
C£®¼ÓČČŅ»¶ØÖŹĮæµÄĄėĆĢĖį¼Ų
D£®ĻņŅ»¶ØĮæµÄĒāŃõ»ÆÄĘČÜŅŗÖŠ¼ÓĖ®Ļ”ŹĶ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĒėøł¾ŻĻĀĮŠ×°ÖĆ»Ų“šĪŹĢā”£
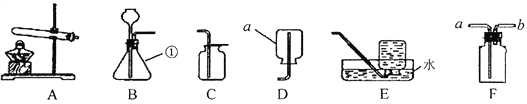
(1)Š“³öĶ¼ÖŠ±źŗÅ¢ŁµÄŅĒĘ÷Ćū³Ę_________”£
(2)ŹµŃéŹŅÓĆøßĆĢĖį¼ŲÖĘŃõĘųµÄ»Æѧ·½³ĢŹ½ĪŖ_________£¬Ń”ŌńµÄ·¢Éś×°ÖĆŹĒ_________(Ģī×°ÖĆŠņŗÅ)£¬ČōŅŖŹÕ¼ÆŅ»Ę湩ĢśĖæČ¼ÉÕŹµŃéÓƵÄŃõĘų£¬Ń”Ōń_________(Ģī×°ÖĆŠņŗÅ)×°ÖĆŹÕ¼ÆŃõĘų±Č½Ļ·½±ćæģ½Ż”£
(3)ŹµŃéŹŅÓĆŠæĮ£ŗĶĻ”ŃĪĖįÖĘĒāĘų£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________£¬ČōŅŖĄūÓĆF×°ÖĆŹÕ¼ÆĒāĘų£¬ŌņĘųĢåÓ¦“Ó_________¶ĖĶØČė;ČōŅŖ¼ģŃéĒāĘųÖŠŹĒ·ńŗ¬ÓŠĀČ»ÆĒāĘųĢ壬æÉŃ”ÓĆĻĀĮŠČÜŅŗÖŠµÄ_________ČÜŅŗ½ųŠŠ¼ģŃ锣
A. NaOHČÜŅŗ B.×ĻÉ«ŹÆČļŹŌŅŗ C. AgNO3ČÜŅŗ D.·ÓĢŖŹŌŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠĶ¼Ļó·Ö±šÓėŃ”ĻīÖŠµÄ²Ł×÷Ļą¶ŌÓ¦£¬ĘäÖŠ²»ŗĻĄķµÄŹĒ

A. ĻņŅ»¶ØĮæµÄ±„ŗĶŹÆ»ŅĖ®ÖŠ¼ÓČėÉśŹÆ»Ņ
B. ĻņĻõĖį±µŗĶĒāŃõ»Æ¼ŲµÄ»ģŗĻČÜŅŗÖŠµĪ¼ÓĻ”ĮņĖį
C. ĻņŅ»¶ØĮæĀČ»ÆøĘŗĶŃĪĖįµÄ»ģŗĻČÜŅŗÖŠµĪ¼ÓĢ¼ĖįÄĘČÜŅŗ
D. ĻņŅ»¶ØĮæµÄĻõĖįŅų”¢ĻõĖįĀĮŗĶĻõĖįĶµÄ»ģŗĻČÜŅŗÖŠ¼ÓČėŠæ·Ū
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com