
| ��������ijԪ�ص��������� |
| ��������ijԪ�ص��������� |
| 14��2 |
| 80 |
| 14 |
| 14+1��5+12+16��3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
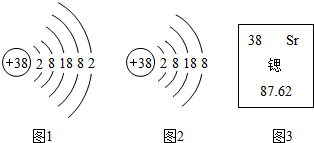
| A��ͼ3����ʾ���ӵĻ�ѧ������Sr |
| B��ͼ2��ͼ3��������Ԫ�� |
| C����ԭ�ӵ�������Ϊ38 |
| D����ԭ�ӵ����ԭ��������87.62 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����pH��ֽ�ⶨ��Һ����ʱ���Ƚ�pH��ֽ��ˮ��ʪ��Ȼ���ٲⶨ |
| B���ڴ����ᴿʵ���У������ᾧֱ����Һ����ʱֹͣ���� |
| C���ø��������ȡ�����������Ƚ��ƾ���Ϩ���ٰѵ��ܴ�ˮ�����Ƴ� |
| D������ϡ����ʱ����Ũ�������ձ�����������ˮ�У������Ͻ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | A | B | C | D |
| ��Ӧǰ��������g�� | 0.6 | 3.8 | 1.0 | 0.6 |
| ��Ӧ���������g�� | ���� | 1.0 | 4.4 | 0 |
| A���÷�Ӧ����ѭ�����غ㶨�� |
| B���÷�Ӧ�ɱ�ʾΪA+B+D��C |
| C��A�����ڷ�Ӧ�в����κ����� |
| D��B��D�����ǵ��ʣ�Cһ���ǻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com