

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaCl�Ȼ���ʳ�� | B��Ca0��������ʯ�� |
| C��Na2CO3̼���ƴ��� | D��NaOH���������ռ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��H2O2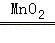 H2O+O2�� H2O+O2�� |
| B��HCl+NaOH=NaCl+H2O |
| C��2Al+O2="2AlO" |
| D��2H2O=2H2+O2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����Ԫ��ԭ������Ϊ8 | B����ԭ�Ӻ���������Ϊ16 |
| C����Ԫ�����ڽ���Ԫ�� | D����Ԫ���ڵؿǵĺ���Ϊ16% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
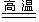 N2��+Cl2��+2O2��+4X����
N2��+Cl2��+2O2��+4X�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO2+2NaOH===Na2CO3+H2O | B��HCl + NaOH===NaCl+H2O |
| C��CaO+H2O===Ca(OH)2 | D��S+O2 SO2 SO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
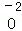 ��_________����
��_________�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com