
| 106 |
| x |
| 44 |
| 2.2g |
| 20g-5.3g |
| 20g |


科目:初中化学 来源: 题型:
 (2010?永州)如图所示,五环中每一个环中有一种物质,相交环表示相应环中的物质问可以相互反应,根据图中信息,请回答:
(2010?永州)如图所示,五环中每一个环中有一种物质,相交环表示相应环中的物质问可以相互反应,根据图中信息,请回答:查看答案和解析>>
科目:初中化学 来源: 题型:
| 实验步骤 | 实验现象 | 结论 |
| ①取少量样品于试管中加适量水,用手触摸试管外壁 | 有热感 | 样品中一定有 (填化学式) |
| ②取少量样品的水溶液于试管中,滴入几滴酚酞试液 | 溶液呈红色 | 样品中含有Ca(OH)2 |
查看答案和解析>>
科目:初中化学 来源: 题型:阅读理解
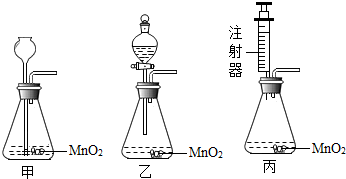 (2010?永州)某校研究性学习小组对采用分解过氧化氢溶液制取氧气进行了实验探究.
(2010?永州)某校研究性学习小组对采用分解过氧化氢溶液制取氧气进行了实验探究.| 实验① | 在试管中加入5mL 5%的过氧化氢溶液 |
| 实验② | 在试管中加入5mL 5%的过氧化氢溶液,并微微加热 |
| 实验③ | 在试管中加入5mL 5%的过氧化氢溶液,并加入少量MnO2 |
查看答案和解析>>
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com