
 =
=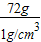 =72cm3=72mL��
=72cm3=72mL�� ��x=73g
��x=73g

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013����б�ҵ��ѧ���ԣ��������¾�����ѧ�������棩 ���ͣ�������
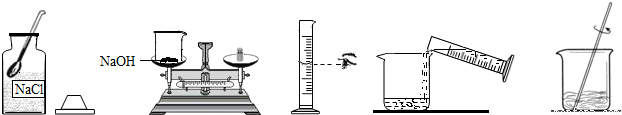
С����Ҫ����80g��������Ϊ10%������������Һ��������Ҷ����ǩ������ͼ�����Ƹ�����������Һ��ʵ�����ʾ��ͼ��
��ʵ�鲽�衿
��1�����㣺��Ҫ�������ƹ����� g��ˮ�� ��mL��ˮ���ܶ���1.0g/cm3�ƣ�
��2����������������ƽ��ȡ�������ƹ��壬�ù��Ϊ�� �����10mL������50mL����100mL��������Ͳȡ����Ҫ��ˮ������ʢ���������Ƶ��ձ��У�
��3���ܽ⣺�ò��������裬ʹ�������ƹ�����ȫ�ܽ⣮
����չ˼ά��
����С�������������Ƶ�����������Һ��ȫ�к�������������Ϊ10%�����ᣬ������������������Ƕ��٣�����Ӧ�Ļ�ѧ����ʽΪ��NaOH+HCl=NaCl+H2O��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
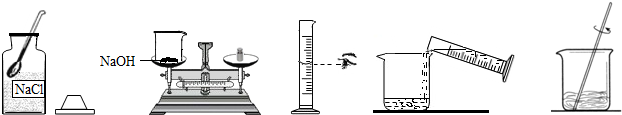
С����Ҫ����80g��������Ϊ10%������������Һ��������Ҷ����ǩ������ͼ�����Ƹ�����������Һ��ʵ�����ʾ��ͼ��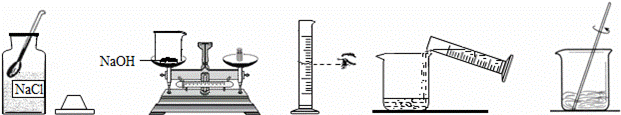
��ʵ�鲽�衿
��1�����㣺��Ҫ�������ƹ�������g��ˮ����mL��ˮ���ܶ���1.0g/cm3�ƣ�
��2����������������ƽ��ȡ�������ƹ��壬�ù��Ϊ�������10mL������50mL����100mL��������Ͳȡ����Ҫ��ˮ������ʢ���������Ƶ��ձ��У�
��3���ܽ⣺�ò��������裬ʹ�������ƹ�����ȫ�ܽ⣮
����չ˼ά��
����С�������������Ƶ�����������Һ��ȫ�к�������������Ϊ10%�����ᣬ������������������Ƕ��٣�
����Ӧ�Ļ�ѧ����ʽΪ��NaOH+HCl=NaCl+H2O��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com