
ʵ�����е��Լ�һ��Ҫ�ܷⱣ�棬������ܻ�������Ӵ������ʡ�ij�о���ѧϰС�鷢��һƿδ�ܱյ�KOH���壬����ɷ�������¼��裬�������ʵ��̽����
����1��ֻ��KOH�� ����2����KOH��K2CO3�� ����3��ֻ��K2CO3
��1���ɷ��п��ܺ���K2CO3��ԭ���ǣ��û�ѧ����ʽ�ش�________________________��
��2��ȡ������Ʒ���Թ��У���������ϡ���ᣬ�۲쵽_____________________��˵������2�����3������
��3����һ��̽���Ĺ������£�
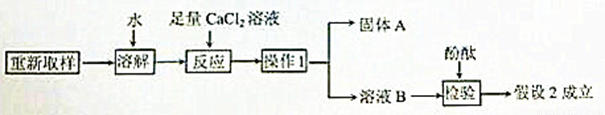
�١�����1����������____________��
�ڡ�����A���Ļ�ѧʽ��___________��
�ۼ�������CaCl2��Һ��������_______________________________��
��4�������Լ�Ҳ�����ü�ֵ����KOH��K2CO3����ɺ����ʿ�����ƿ�����Լ�����;��
__________________________________________��д��һ������
��1��2KOH + CO2 === K2CO3 + H2O (2��)
(2)�����ݲ��� ��1�֣�
(3)�ٹ��� ��1�֣� ��CaCO3 (1��)
�� ��̼�����ȫ��Ӧ����ֹ�Խ�������ʵ����� ��2�֣�
(4)���طʣ������𰸺�����ɣ���1�֣�
����������1��KOH���ڼ�ڿ����з��ÿ�������еĶ�����̼���巢����Ӧ���Ӷ����ʣ�����̼���κ�ˮ���� ���Է�Ӧ�Ļ�ѧ����ʽΪ��2KOH+ CO2 === K2CO3 + H2O
��2��ϡ�������K2CO3��Ӧ���ɶ�����̼���壬���������2�����3����������K2CO3��������ϡ���������Ӧ��Ϊ�������ݲ�����
��3���١�����1���������ǽ�CaCl2 ��Һ����Ʒ����Һ��Ӧ���������ֳ��˹�����Һ�壬�����������Һ�壬���Դ˲���ӦΪ���ˡ�
�ڡ�����A����һ�ֲ�����ˮ�����ʣ��ۺ�ǰ��IJ�����жϳ���Ʒ�ijɷ��к���K2CO3������A��K2CO3��CaCl2��Ӧ���ɵ�̼��ƣ���ѧʽΪCaCO3��
��CaCl2����K2CO3��Ӧ��������CaCl2���㣬����K2CO3ȫ����Ӧ��������ҺB�н�����������K2CO3��K2CO3�ʼ��ԣ���ʹ��̪��졣��������֤KOH�Ƿ���ڡ����Լ���������CaCl2��Ŀ���ǣ���̼�����ȫ��Ӧ����ֹ�Խ�������ʵ�飨��֤KOH�Ƿ���ڣ����š�
��4��KOH��K2CO3������Ԫ�أ����Կ�������ȡ�طʡ�
���㣺��ѧ̽������Ļ�ѧ���ʡ����ʵ��ƶ�
�������������˼��������̼�ķ�Ӧ�����ü�ı��ʿ���������ʵ����֤���뷽���Ƿ������Ӧע����Ƿ�ȫ�����ʡ���ȫ�����ʣ���ɷ�ΪK2CO3���粿�ֱ��ʣ���ɷ�ΪKOH��K2CO3��


 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013����б�ҵ��ѧ���ԣ��㶫���ݾ�����ѧ���������� ���ͣ��ʴ���
ʵ�����е��Լ�һ��Ҫ�ܷⱣ�棬������ܻ�������Ӵ������ʡ�ij�о���ѧϰС�鷢��һƿδ�ܱյ�KOH���壬����ɷ�������¼��裬�������ʵ��̽����
����1��ֻ��KOH�� ����2����KOH��K2CO3�� ����3��ֻ��K2CO3
��1���ɷ��п��ܺ���K2CO3��ԭ���ǣ��û�ѧ����ʽ�ش�________________________��
��2��ȡ������Ʒ���Թ��У���������ϡ���ᣬ�۲쵽_____________________��˵������2�����3������
��3����һ��̽���Ĺ������£� 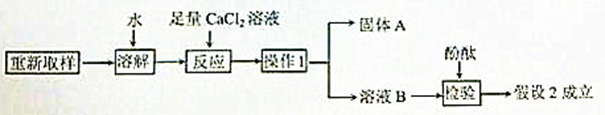
�١�����1����������____________��
�ڡ�����A���Ļ�ѧʽ��___________��
�ۼ�������CaCl2��Һ��������_______________________________��
��4�������Լ�Ҳ�����ü�ֵ����KOH��K2CO3����ɺ����ʿ�����ƿ�����Լ�����;��
__________________________________________��д��һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㶫ʡ�п����� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��㶫ʡ�������п���ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com