
С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���塣
ʵ���������ݼ��±�����������������йؼ��㣺
| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
��1����Ʒ��̼���Ƶ����������Ƕ��٣�(�����ȷ��0.1%)
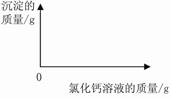
��2������ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע����ɫ�������Ȼ�����Һ�������������


 53������ϵ�д�
53������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?̩����С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮
��2012?̩����С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�п����� ���ͣ�������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮
ʵ���������ݼ��±�����������������йؼ��㣺
| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
��1����Ʒ��̼���Ƶ����������Ƕ��٣��������ȷ��0.1%��
��2������ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע��
��ɫ�������Ȼ�����Һ�������������

| |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ��������ˮ�����Һ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���塣
ʵ���������ݼ��±�����������������йؼ��㣺
| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
��1����Ʒ��̼���Ƶ����������Ƕ��٣�(�����ȷ��0.1%)
��2������ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע����ɫ�������Ȼ�����Һ�������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com