��2008?��������ģ��ʵ�����д�ŵ��������ƹ��峣�������������̼���ƣ�С���ʹ�����չ����̽����
�������ռ���
��1���������ƿ���������е�
������̼
������̼
��Ӧ������̼���ƣ�̼����ˮ��Һ�Լ��ԣ���ʹ��ɫ��̪��Һ��죮
��2���ڳ��¡�101kPa��CO
2���ܶ�Ϊ1.8g/L��
��ʵ�鷽��1��Ŀ�ģ�����NaOH��Ʒ���Ƿ����Na
2CO
3��
С�����������ʵ�鷽�������������ʵ�鷽���ͽ�����һ��ǡ�������ۣ���˵���������л��У���˵��ԭ��
|
ʵ�鷽�� |
��ʵ�鷽�������� |
| ����1 |
��������Ʒ��������ˮ����������̪��Һ�����ݷ�̪��Һ�Ƿ��죬�ж��Ƿ���̼���� |
|
| ����2 |
��������Ʒ��������ˮ��������ϡ������Һ�������Ƿ�������ݣ��ж��Ƿ���̼���� |
|
| ����3 |
��������Ʒ��������ˮ���������Ȼ�����Һ�������Ƿ������ɫ�������ж��Ƿ���̼���� |
|
��ʵ�鷽��2��Ŀ�ģ��ⶨNaOH��Ʒ��Na
2CO
3������������
С����ʵ�鲽�����£�װ�ú�ҩƷ��ͼ��ʾ����
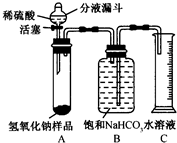
��Һ©����������ϡ��������Թ������������رջ�������Ӧ��������Ͳ���ռ�������NaHCO
3��Һ220mL�������С����ʵ������ܽ
��1����֪B�м���ƿʢװ�ı���NaHCO
3��Һ������ˮ���棬���Ʋ���������
������̼������ˮ�������ڱ���NaHCO3��Һ���������̼�ڱ���NaHCO3��Һ�е��ܽ�Ⱥ�С�����������Ա����������̼����ˮ��ɵIJ������
������̼������ˮ�������ڱ���NaHCO3��Һ���������̼�ڱ���NaHCO3��Һ�е��ܽ�Ⱥ�С�����������Ա����������̼����ˮ��ɵIJ������
��
��2����Ʒ�к��е�̼���Ƶ�����Ϊ
0.954
0.954
g��
��3��Ϊ�ﵽʵ��Ŀ�ģ���ʵ�黹ȱ�ٵ�һ��ʵ�������
�ⶨ��ȡ��Ʒ������
�ⶨ��ȡ��Ʒ������
��
��4����ʵ���г������������⣬���жԲⶨNaOH��Ʒ��Na
2CO
3������������Ӱ����ǣ�����ţ�
�٢�
�٢�
��
�ٳ���ǰ���������ƹ����ڿ����з�������
��װ��A�м����ϡ���᱾��Ҳռ��һ�������
������������Ʒ�г���̼�����������⣬�����������Ȼ��ƣ�

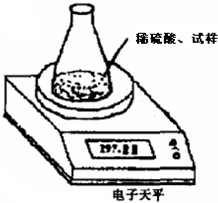 ��ͼװ�òⶨ���Ų��������ֱ��̼���Ƶ��ռ����������Ƶ�������������ȡ��������8.00g����ƿ����140.00g����������ϡ���ᣨ����Ϊ50.00g��ÿ����ͬʱ�����һ�Σ��������±���
��ͼװ�òⶨ���Ų��������ֱ��̼���Ƶ��ռ����������Ƶ�������������ȡ��������8.00g����ƿ����140.00g����������ϡ���ᣨ����Ϊ50.00g��ÿ����ͬʱ�����һ�Σ��������±���


