
[ŃŠ¾æ·½°ø] ĻČ³ĘČ”13.3gµÄNaOHѳʷ£ØŌÓÖŹĪŖNa2CO3£©£¬Åä³ÉČÜŅŗ£¬Č»ŗóĻņČÜŅŗÖŠÖšµĪ¼ÓČėÖŹĮæ·ÖŹżĪŖ14.6%µÄĻ”ŃĪĖį£¬øł¾ŻÉś³ÉCO2µÄÖŹĮæ²ā¶ØNa2CO3µÄÖŹĮ攣“Ó¶ų½ųŅ»²½Č·¶Øѳʷ֊NaOHµÄ±äÖŹ³Ģ¶Č”£
[½ā¾öĪŹĢā] ŹµŃé²āµĆ¼ÓČėĻ”ŃĪĖįµÄÖŹĮæÓė²śÉśCO2ĘųĢåµÄÖŹĮæ¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£
|
Na2CO3µÄÖŹĮæ/g |
|
|
±äÖŹNaOHµÄÖŹĮæ/g |
|
|
NaOHµÄ±äÖŹ³Ģ¶Č£ØÓĆÖŹĮæ·ÖŹż±ķŹ¾£© |
|
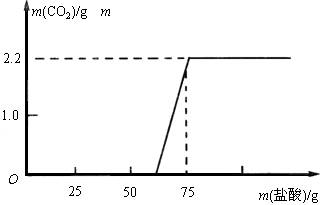
ĢīŠ“ÉĻ±ķ£ŗ£Ø¼ĘĖć½į¹ū±£ĮōŠ”ŹżµćŗóŅ»Ī»£©
[¼ĢŠųĢ½¾æ] ĒóŹµŃé¹ż³ĢÖŠÓėNaOH·“Ó¦ĖłÓĆŃĪĖįµÄÖŹĮ攣
[·¢ĻÖĪŹĢā] øł¾Ż”°ÓėNaOH·“Ó¦ĖłÓĆŃĪĖįµÄÖŹĮæ”±£¬¶ŌÕÕĶ¼Ļó£¬Äć·¢ĻÖĮĖŹ²Ć“ĪŹĢā?
[½ā¾öĪŹĢā]
[¼ĢŠųĢ½¾æ] m((NaOH))=13.3g-5.3g=8g NaOH+HClØTNaCl+H2O 40 36.5 8g m(HCl) m(HCl)=8g´ m[HCl(aq)]= [·¢ĻÖĪŹĢā] NaOH±»ÖŠŗĶŗ󣬵Ī¼ÓŃĪĖį£¬ĪŖŹ²Ć“ƻӊĮ¢¼“²śÉśCO2ĘųĢå?£ØĘäĖūŗĻĄķ“š°øŅ²øų·Ö£©
|
| Ļ”ŃĪĖįŹ×ĻČŗĶĒāŃõ»ÆÄĘ·“Ó¦£¬Ć»ÓŠ³ĮµķÉś³É£¬µ±ÖŠŗĶĶźČ«ŗ󣬲ÅŗĶĢ¼ĖįÄĘ·“Ó¦·Å³ö¶žŃõ»ÆĢ¼ĘųĢ唣
|


 ÖĒ»ŪŠ”ø“Ļ°ĻµĮŠ“š°ø
ÖĒ»ŪŠ”ø“Ļ°ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 34”¢Ä³ŃŠ¾æŠŌѧĻ°Š”×éĢ½¾æCuSO4ČÜŅŗÓėNaOHČÜŅŗµÄ·“Ó¦²śĪļ£®
34”¢Ä³ŃŠ¾æŠŌѧĻ°Š”×éĢ½¾æCuSO4ČÜŅŗÓėNaOHČÜŅŗµÄ·“Ó¦²śĪļ£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
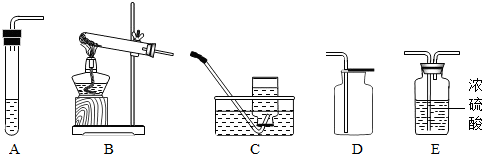
| ÖĘČ”ĘųĢå | ĖłŠčŅŖĘ· | ×°ÖĆĮ¬½ÓĖ³Šņ | ·“Ó¦µÄ»Æѧ·½³ĢŹ½ |
| ŃõĘų | ĀČĖį¼ŲŗĶ¶žŃõ»ÆĆĢ | ||
| ¶žŃõ»ÆĢ¼£ØøÉŌļ£© | “óĄķŹÆŗĶĻ”ŃĪĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| Na2CO3µÄÖŹĮæ/g | 5.3 5.3 |
| ±äÖŹNaOHµÄÖŹĮæ/g | 4 4 |
| NaOHµÄ±äÖŹ³Ģ¶Č | 33.3% 33.3% |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
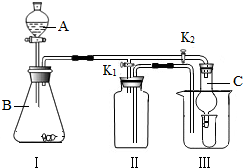
Ä³ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§ÓūĄūÓĆĻĀĮŠ×°ÖĆĢ½¾æĀĢÉ«Ö²ĪļŗōĪü¹ż³ĢÖŠŹĒ·ńÓŠ
CO2ĘųĢå²śÉś£¬Éč¼ĘµÄĢ½¾æ¹ż³ĢČēĻĀ£¬Ēė»Ų“šĘäÖŠµÄÓŠ¹ŲĪŹĢā£®

£Ø1£©¼ŁÉč£ŗĀĢÉ«Ö²ĪļŌŚŗōĪü¹ż³ĢÖŠÓŠCO2ĘųĢå²śÉś”£
£Ø2£©Éč¼Ę·½°ø£ŗŹ¹ĀĢÉ«Ö²ĪļŌŚ±Ü¹āµÄŗŚ°µ“¦·¢ÉśŗōĪü×÷ÓĆ£¬¼ģŃéŗōĪü¹ż³ĢÖŠ²śÉśµÄĘųĢ壮
£Ø3£©²éŌÄ׏ĮĻ£ŗ¢ŁĀĢÉ«Ö²Īļ¹āŗĻ×÷ÓĆ¹ż³Ģ£ŗĖ®+¶žŃõ»ÆĢ¼Ņ»”śÓŠ»śĪļ+ŃõĘų
¢ŚĀĢÉ«Ö²ĪļŗōĪü×÷ÓĆ¹ż³Ģ£ŗÓŠ»śĪļ+ŃõĘųŅ»”ś¶žŃõ»ÆĢ¼+Ė®+ÄÜĮæ
£Ø4£©ŹµŃé£ŗ
| ²Ł×÷²½Öč | ¼ņ“š |
| ¢Ł ½«ø÷×°ÖĆ°“ÉĻĶ¼ ĖłŹ¾Į¬½ÓŗĆ²¢×°Čė»ÆѧŹŌ¼Į£® CÖŠ·ÅČėĀĢÉ«Ö²Īļ | ¢Ł A×°ÖƵÄ×÷ÓĆŹĒ ¢Ś B×°ÖƵÄ×÷ÓĆŹĒ ¢Ū C“¦²£Į§ÕÖ²»ÄÜĶø¹āµÄŌŅņŹĒ________________
|
| ¢Ś ĶłAµÄµ¼¹ÜæŚ»ŗ»ŗĮ¬Šų ¹ÄČėæÕĘųŅ»¶ĪŹ±¼ä | ¢Ü A×°ÖĆÖŠÓ¦¹Ū²ģµ½µÄĻÖĻóŹĒŹÆ»ŅĖ®±ä»ė×Ē ¢ŻD×°ÖĆÖŠÓ¦¹Ū²ģµ½µÄĻÖĻóŹĒŹÆ»ŅĖ®±ä»ė×Ē |
£Ø5£©½įĀŪÓėĢÖĀŪ£ŗ¢ŁÅŠ¶ĻÕāøöĢ½¾æŹµŃé________£ØĢī”°³É¹¦”±»ņ”°²»³É¹¦”±£©£»
¢ŚČē¹ūøĆŃŠ¾æŠŌѧĻ°Š”×éĢ½¾æŹµŃé²»³É¹¦£¬ŌŅņŹĒ¶ą·½ĆęµÄ”£ĒėÄć“ÓŹµŃéĢõ¼žæŲÖĘÉĻÕŅ³öæÉÄܵÄŅ»øöŌŅņ£ŗ________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ä³ŃŠ¾æŠŌѧĻ°Š”×éµÄĶ¬Ń§ÓūĄūÓĆĻĀĮŠ×°ÖĆĢ½¾æĀĢÉ«Ö²ĪļŗōĪü¹ż³ĢÖŠŹĒ·ńÓŠ
CO2ĘųĢå²śÉś£¬Éč¼ĘµÄĢ½¾æ¹ż³ĢČēĻĀ£¬Ēė»Ų“šĘäÖŠµÄÓŠ¹ŲĪŹĢā£®

£Ø1£©¼ŁÉč£ŗĀĢÉ«Ö²ĪļŌŚŗōĪü¹ż³ĢÖŠÓŠCO2ĘųĢå²śÉś”£
£Ø2£©Éč¼Ę·½°ø£ŗŹ¹ĀĢÉ«Ö²ĪļŌŚ±Ü¹āµÄŗŚ°µ“¦·¢ÉśŗōĪü×÷ÓĆ£¬¼ģŃéŗōĪü¹ż³ĢÖŠ²śÉśµÄĘųĢ壮
£Ø3£©²éŌÄ׏ĮĻ£ŗ¢ŁĀĢÉ«Ö²Īļ¹āŗĻ×÷ÓĆ¹ż³Ģ£ŗĖ®+¶žŃõ»ÆĢ¼Ņ»”śÓŠ»śĪļ+ŃõĘų
¢ŚĀĢÉ«Ö²ĪļŗōĪü×÷ÓĆ¹ż³Ģ£ŗÓŠ»śĪļ+ŃõĘųŅ»”ś¶žŃõ»ÆĢ¼+Ė®+ÄÜĮæ
£Ø4£©ŹµŃé£ŗ
| ²Ł×÷²½Öč | ¼ņ“š |
| ¢Ł ½«ø÷×°ÖĆ°“ÉĻĶ¼ ĖłŹ¾Į¬½ÓŗĆ²¢×°Čė»ÆѧŹŌ¼Į”£CÖŠ·ÅČėĀĢÉ«Ö²Īļ | ¢Ł A×°ÖƵÄ×÷ÓĆŹĒ ¢Ś B×°ÖƵÄ×÷ÓĆŹĒ ¢Ū C“¦²£Į§ÕÖ²»ÄÜĶø¹āµÄŌŅņŹĒ________________
|
| ¢Ś ĶłAµÄµ¼¹ÜæŚ»ŗ»ŗĮ¬Šų ¹ÄČėæÕĘųŅ»¶ĪŹ±¼ä | ¢Ü A×°ÖĆÖŠÓ¦¹Ū²ģµ½µÄĻÖĻóŹĒŹÆ»ŅĖ®±ä»ė×Ē ¢Ż D×°ÖĆÖŠÓ¦¹Ū²ģµ½µÄĻÖĻóŹĒŹÆ»ŅĖ®±ä»ė×Ē |
£Ø5£©½įĀŪÓėĢÖĀŪ£ŗ¢ŁÅŠ¶ĻÕāøöĢ½¾æŹµŃé________£ØĢī”°³É¹¦”±»ņ”°²»³É¹¦”±£©£»
¢ŚČē¹ūøĆŃŠ¾æŠŌѧĻ°Š”×éĢ½¾æŹµŃé²»³É¹¦£¬ŌŅņŹĒ¶ą·½ĆęµÄ”£ĒėÄć“ÓŹµŃéĢõ¼žæŲÖĘÉĻÕŅ³öæÉÄܵÄŅ»øöŌŅņ£ŗ________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com