
ŹµŃéŹŅÓŠŅ»Ęæ¾ĆÖƵÄNaOH£¬³ĘČ”13.3g µÄNaOHѳʷ£ØŌÓÖŹĪŖNa2CO3£©ÓŚÉÕ±ÖŠ£¬Č»ŗóĻņÉÕ±ÖŠÖšµĪ¼ÓČėÖŹĮæ·ÖŹż19.6%µÄĻ”ĮņĖį£¬·“Ó¦ĒéæöČēĶ¼ĖłŹ¾”£

£Ø1£©ŹŌ¼ĘĖć£ŗ
¢ŁøĆѳʷ֊Na2CO3µÄÖŹĮæĪŖ¶ąÉŁ£æ
¢ŚBµćĖł¶ŌÓ¦µÄČÜŅŗÖŠČÜÖŹµÄÖŹĮæ·ÖŹżĪŖ¶ąÉŁ£æ
£Ø2£©“ÓĶ¼ÖŠ0”«AµćĖµĆ÷:ŌŚNaOH ÓėNa2CO3µÄ»ģŗĻČÜŅŗÖŠ£¬¼ÓČėĒæĖį£¬Ź×ĻČ·“Ó¦µÄĪļÖŹŹĒ £»¢Śøł¾Ż·“Ó¦·½³ĢŹ½·ÖĪö£¬NaOH²æ·Ö±äÖŹ»ņČ«²æ±äÖŹ£¬Óėƻӊ±äÖŹµÄNaOHĻą±Č£¬ÖŠŗĶ·“Ó¦Ź±ĻūŗÄĒæĖįµÄĮæ (Ģī”°ĻąµČ”±”¢”°²»ĻąµČ”±»ņ”°ĪŽ·ØČ·¶Ø”±)”£
 ŗ®¼ŁĄÖŌ°±±¾©½ĢÓż³ö°ęÉēĻµĮŠ“š°ø
ŗ®¼ŁĄÖŌ°±±¾©½ĢÓż³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
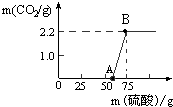 ŹµŃéŹŅÓŠŅ»Ęæ¾ĆÖƵÄNaOH£¬³ĘČ”13.3g µÄNaOHѳʷ£ØŌÓÖŹĪŖNa2CO3£©ÓŚÉÕ±ÖŠ£¬Č»ŗóĻņÉÕ±ÖŠÖšµĪ¼ÓČėÖŹĮæ·ÖŹż19.6%µÄĻ”ĮņĖį£¬·“Ó¦ĒéæöČēĶ¼ĖłŹ¾£®
ŹµŃéŹŅÓŠŅ»Ęæ¾ĆÖƵÄNaOH£¬³ĘČ”13.3g µÄNaOHѳʷ£ØŌÓÖŹĪŖNa2CO3£©ÓŚÉÕ±ÖŠ£¬Č»ŗóĻņÉÕ±ÖŠÖšµĪ¼ÓČėÖŹĮæ·ÖŹż19.6%µÄĻ”ĮņĖį£¬·“Ó¦ĒéæöČēĶ¼ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
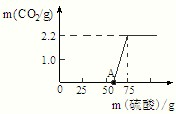 ijŠ£ŃŠ¾æŠŌѧĻ°Š”×é½ųŠŠĮĖŅ»øöӊȤµÄŹµŃéĢ½¾æ£ŗ
ijŠ£ŃŠ¾æŠŌѧĻ°Š”×é½ųŠŠĮĖŅ»øöӊȤµÄŹµŃéĢ½¾æ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŹµŃéŹŅÓŠŅ»Ęæ¾ĆÖƵÄĒāŃõ»ÆÄĘ¹ĢĢ壬ijĶ¬Ń§ĻėĮĖ½āĖüŹĒ·ń±äÖŹ£¬Éč¼ĘĮĖŅŌĻĀŹµŃ飬ĒėÄćÓėŅ»ĘšĶź³ÉŅŌĻĀĢ½¾æ»ī¶Æ£ŗ
ŹµŃéŹŅÓŠŅ»Ęæ¾ĆÖƵÄĒāŃõ»ÆÄĘ¹ĢĢ壬ijĶ¬Ń§ĻėĮĖ½āĖüŹĒ·ń±äÖŹ£¬Éč¼ĘĮĖŅŌĻĀŹµŃ飬ĒėÄćÓėŅ»ĘšĶź³ÉŅŌĻĀĢ½¾æ»ī¶Æ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ČÜÓŚĖ®Åä³ÉČÜŅŗ |
| ¼ÓČė¹żĮæµÄCaCl2ČÜŅŗ |
| ¹żĀĖ”¢Ļ“µÓ |
| øÉŌļ”¢³ĘĮæ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com