
¹¤ŅµÉĻÓĆĀĮĶĮæó£Øŗ¬Ńõ»ÆĀĮ”¢Ńõ»ÆĢś£©ÖĘČ”ĀĮµÄ¹ż³ĢČēĻĀ£ŗ
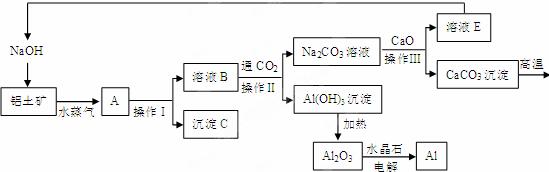
”¾²éŌÄ׏ĮĻ”æ1”¢Ńõ»ÆĀĮŗĶĒāŃõ»ÆÄĘČÜŅŗÄÜ·¢Éś»Æѧ·“Ó¦£¬¶ųŃõ»ÆĢś²»ÄÜŗĶĒāŃõ»ÆÄĘ·“Ó¦
2Ӣ2NaOH + Al2O3==2NaAlO2 + H2O
Ēėøł¾ŻÉĻŹöĮ÷³ĢĢį¹©µÄŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
1£®²Ł×÷¢ņµÄĆū³ĘŹĒ__________”£
2. ³ĮµķCµÄ»ÆѧŹ½ŹĒ___________£¬ŹµŃéŹŅÓĆŃĪĖį½«ĘäČܽāµĆµ½________É«µÄČÜŅŗ”£
3.Éś²ś¹ż³ĢÖŠ£¬³żH2OæÉŅŌŃ»·Ź¹ÓĆĶā£¬»¹æÉŅŌŃ»·Ź¹ÓƵÄĪļÖŹÓŠ________”£
A.¶žŃõ»ÆĢ¼  B.Ńõ»ÆøĘ C.ĒāŃõ»ÆÄĘ
B.Ńõ»ÆøĘ C.ĒāŃõ»ÆÄĘ
4.Š“³öČÜŅŗBŗĶ¶žŃõ»ÆĢ¼·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½___ _______________________________
_______________________________

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| ||
| ||
 £¬Ęä×īĶā²ćµē×ÓŹżŹĒ
£¬Ęä×īĶā²ćµē×ÓŹżŹĒ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2011ÄźÉĀĪ÷Ź”ÖŠæ¼Ąķ»ÆÄ£ÄāŹŌ¾ķ»Æѧ²æ·Ö£ØŅ»£©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com