
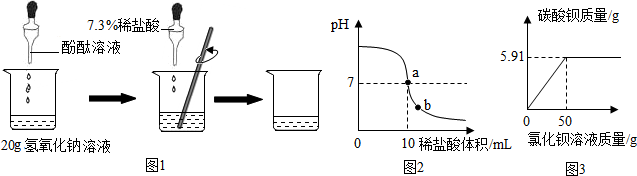
���� ��1���ٸ��ݷ�̪��������Һ��죬����������������Ƿ����кͷ�Ӧ��
�ڸ��ݲ����������÷����ش�
�۸�����������������ķ�Ӧ�����������ʵ���������������Ƶ����������������������Һ�����ʵ�����������
��2���ٸ���pH��ֽʹ�÷��������жϣ�
�ڸ�����ҺpH�ı仯ͼ������壬����a�ĺ��塢b����Һ�е������ӣ��������������ܶȿ���������������
��3���ٸ������������������̼�ķ�Ӧд���������Ʊ��ʵķ���ʽ������̼���Ƶ����ʷ�����Ƴ�ȥ̼���Ƶ�ʵ�鷽�����ڸ�������̼�ᱵ����������̼���Ƶ���������һ���������Һ��̼���Ƶ�����������
��� �⣺��1���ٷ�̪��������Һ��죬��̪��Һ�������ǣ��жϷ�Ӧ�Ƿ�ǡ����ɣ�
�����кͷ�Ӧ�����У��ߵμ�ϡ���ᣬ��Ҫ�ò��������Ͻ����Ŀ���ǣ�ʹ��Ӧ���ֽӴ�����ȫ��Ӧ��
�����������ʵ�����Ϊ��7.3%��10g=0.73g
���������Ƶ�����Ϊx
NaOH+HCl=NaCl+H2O
40 36.5
x 0.73g
$\frac{40}{x}=\frac{36.5}{0.73g}$
��ã�x=0.8g
������ƿ����������Һ�����ʵ���������Ϊ��$\frac{0.8g}{20g}$��100%=4%��
��2����A����pH��ֽ���ɼ��ν�Լʹ�ã�������ȷ��
B����pH��ֱֽ�Ӳ������Һ�У�����Ⱦ�Լ�����������
C����pH��ֽ��ʪ��ü�Һ��pHƫС����������
D����pH��ֽ���ڸɾ��İ״ɰ��ϣ��ò�����պȡ����Һ����pH��ֽ�ϣ�������ȷ��
������ҺpH�ı仯ͼ���֪����a��ʱ����Һ��pH����7��˵�����������ƺ�����ǡ���кͣ���b��ʱ����Һ�����ԣ���������ȫ�������ᷴӦ�������Ȼ��ƣ���Һ�л���ʣ������ᣮ������Һ�е��������ǣ�Na+��H+��Ҫ������������Һ������������������֪����������������е�������֪������Ҫ��������ϡ������ܶȣ�
��3�����������Ʊ��ʵ�ԭ������������������еĶ�����̼��Ӧ����Ӧ�ķ���ʽ�ǣ�CO2+2NaOH=Na2CO3+H2O������̼���������������Ʒ�Ӧ�������������ƺ�̼��ƣ��ȳ�ȥ������̼���ƣ���û�������µ����ʣ����ԣ�Ҫ��ȥ��Һ�б������ɵ����ʣ�ʵ�鷽���ǣ�����������ʯ��ˮ�����ˣ�
����̼���Ƶ�����Ϊy
BaCl2+Na2CO3�TBaCO3��+2NaCl
106 197
x 5.91g
$\frac{106}{x}=\frac{197}{5.91g}$
x=3.18g
������Һ��̼���Ƶ���������Ϊ$\frac{3.18g}{100g}$��100%�T3.2%��
�ʴ�Ϊ����1��ָʾ�������жϷ�Ӧ�ķ����� �ӿ췴Ӧ�ٶȣ� 4%��
��2��AD�� ǡ����ȫ��Ӧ�� H+��Na+�� ϡ������ܶ�
��3����CO2+2NaOH�TNa2CO3+H2O�� Ca��OH��2��Ba��OH��2��
��3.2%��
���� �����ǿ����кͷ�Ӧ��������ҺpH�ı仯����ģ�����Ҫ֪�������мӼ������м���ʱ����ҺpH�ı仯������ȷ��ָͬʾ���ı�ɫ��Χ��


 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
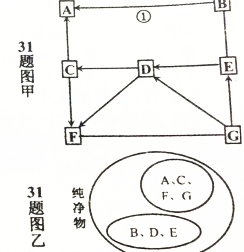 AһG�dz��л�ѧ���������ʣ�����֮���ת����ϵ��ͼ����ʾ�����ַ�Ӧ��������Ӧ�P����������ȥ��C�������������ߵĽ�����F���Ϻ�ɫ������A-G֮��ĸ����ϵ��ͼ����ʾ����ش��������⣺
AһG�dz��л�ѧ���������ʣ�����֮���ת����ϵ��ͼ����ʾ�����ַ�Ӧ��������Ӧ�P����������ȥ��C�������������ߵĽ�����F���Ϻ�ɫ������A-G֮��ĸ����ϵ��ͼ����ʾ����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȼ���̻����� | B�� | ������չ�������� | ||
| C�� | ¶��������� | D�� | ��ߵ綯�����ı��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �ס������ֲ����ᾧˮ�Ĺ������ʵ��ܽ��������ͼ������˵������ȷ���ǣ�������
�ס������ֲ����ᾧˮ�Ĺ������ʵ��ܽ��������ͼ������˵������ȷ���ǣ�������| A�� | ���ܽ�ȱ��Ҵ� | |
| B�� | t2��ʱ���ס��ҵ�������Һ�����ʵ������������ | |
| C�� | Ҫʹ�ӽ����͵�����Һת��Ϊ������Һ���ɲ��������¶ȵķ��� | |
| D�� | t1��ʱ����50gˮ����15g�������У��ɵõ�60g��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ȼ������������ȼ�Ͽɼ��ٿ�����Ⱦ | |
| B�� | �����е��ޡ���ë�ȶ������л��߷��Ӻϳɲ��� | |
| C�� | ����ʹ�ÿɽ������ϣ�����Ч�������ɫ��Ⱦ������ | |
| D�� | ʳ�üӵ�ʳ�κ�ǿ�������Ϳɲ�����������ijЩ��Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com