
ĻĀĶ¼ĖłŹ¾ŹĒŹµŃéŹŅÖŠÖĘČ”ĘųĢåŹ±³£ÓĆ×°ÖĆ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ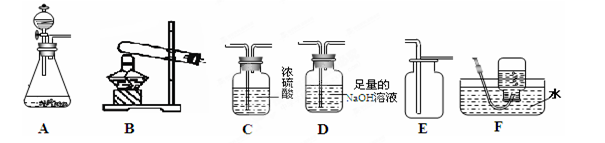
£Ø1£©ŌŚŹµŃéŹŅÖŠÖĘČ”ĘųĢåŹ±£¬¼ÓČėŅ©Ę·Ö®Ē°Ņ»¶ØŅŖĻČ¼ģŃé ”£
£Ø2£©ŅŌŹÆ»ŅŹÆŗĶĻ”ŃĪĖįĪŖŌĮĻÖĘČ”²¢ŹÕ¼ÆøÉŌļµÄCO2ĘųĢ唣
¢Łø÷×°ÖƵÄÕżČ·Į¬½ÓĖ³ŠņŹĒ£ØĢī×°ÖĆŠņŗÅ£© ”£
¢Ś²śÉśCO2ĘųĢåµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
¢ŪC×°ÖƵÄ×÷ÓĆŹĒ ”£
¢Ü¼ģŃé¼ÆĘųĘæÖŠŹĒ²»ŹĒCO2ĘųĢåµÄ·½·Ø ”£
£Ø3£©Ķ¬Ń§¼×ĪŖŃéÖ¤ÖĘČ”CO2ŗóŹ£ÓąČÜŅŗÖŠŹĒ·ńŗ¬ÓŠŃĪĖį£ØŌÓÖŹ²»ČÜÓŚĖ®ĒŅ²»²Ī¼Ó·“Ó¦£¬ĒŅCaCl2ČÜŅŗ³ŹÖŠŠŌ£©£¬Éč¼ĘŹµŃé·½°øČēĻĀ£ŗȔѳӌŹŌ¹ÜÖŠ£¬µĪ¼ÓAgNO3ČÜŅŗ£¬²śÉś°×É«³Įµķ£¬ŌŁ¼ÓČėĻ”ĻõĖį£¬³Įµķ²»Čܽā£¬Ōņŗ¬ÓŠŃĪĖį”£ÄćŹĒ·ńĶ¬ŅāøĆ·½°ø£æ¼ņŹöĄķÓÉ”£ ”£
£Ø4£©½«ÉŁĮæCO2ĘųĢåĶØČėŹ¢ÓŠ×ćĮæCa(OH)2ČÜŅŗµÄ¹ćæŚĘæÖŠ£¬ĘæÖŠČÜŅŗµÄÖŹĮæÓėÖ®Ē°Ļą±Č»į £ØĢīŠ“ĻĀĮŠø÷ĻīŠņŗÅ£©”£
| A£®Ōö“ó | B£®¼õŠ” | C£®²»±ä | D£®ĪŽ·ØÅŠ¶Ļ |
£Ø1£©×°ÖƵÄĘųĆÜŠŌ
£Ø2£©¢ŁACE£»¢ŚCaCO3+2HCl=CaCl2+2H2O+CO2”ü£»¢ŪøÉŌļĘųĢå»ņĪüŹÕĘųĢåÖŠµÄĖ®·Ö£»¢Ü¼ÓČėÉŁĮæµÄ³ĪĒåŹÆ»ŅĖ®²¢Õńµ“£¬Čō擵½³ĪĒåµÄŹÆ»ŅĖ®±ä»ė×Ē£¬ŌņŹĒCO2£»
£Ø3£©²»Ķ¬Ņā£¬ŅņĪŖŹ£ÓąČÜŅŗÖŠŅ»¶Øŗ¬ÓŠCaCl2£¬²»¹ÜŹĒ·ńÓŠĻ”ŃĪĖį£¬µĪ¼ÓAgNO3¶¼»į²śÉś²»ČÜÓŚĻ”ĻõĖįµÄ°×É«³Įµķ£»
£Ø4£©A
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ŌŚŹµŃéŹŅÖĘČ”ĘųĢåŹ±£¬¼ÓČėŅ©Ę·Ö®Ē°Ņ»¶ØŅŖĻČ¼ģŃé×°ÖƵÄĘųĆÜŠŌ£¬ŅŌ·ĄÉś³ÉµÄĘųĢåŅŻ³ö£»£Ø2£©¢ŁŹµŃéŹŅÓĆ“óĄķŹÆŗĶĻ”ŃĪĖį·“Ó¦ÖĘČ”¶žŃõ»ÆĢ¼£¬·“Ó¦ĪļµÄדŹĒ¹ĢĢåŗĶŅŗĢ壬·“Ó¦Ģõ¼žŹĒ³£ĪĀ£¬Ó¦Ń”ÓĆ·¢Éś×°ÖĆA£»ÓÉÓŚ¶žŃõ»ÆĢ¼µÄĆܶȓóÓŚæÕĘųµÄĆÜ¶Č£¬æÉŃ”ÓĆŹÕ¼Æ×°ÖĆB£»ŌŚŹÕ¼ÆĒ°ÓĆÅØĮņĖį½ųŠŠøÉŌļ£¬ĖłŅŌæɲÉÓƵÄ×°ÖĆ×éŗĻŹĒACE£»¢ŚŹµŃéŹŅÖĘČ”CO2ÓĆ“óĄķŹÆŗĶĻ”ŃĪĖį£¬»Æѧ·½³ĢŹ½ĪŖ£ŗCaCO3 +2HClØTCaCl2+H2O+CO2”ü£»¢ŪÅØĮņĖį¾ßÓŠĪüĖ®ŠŌ£¬ĖłŅŌæÉÓĆĄ“øÉŌļĘųĢå»ņĪüŹÕĘųĢåÖŠµÄĖ®·Ö£»¢Ü¼ģŃéCO2ĘųĢåµÄ·½·Ø¼ÓČėÉŁĮæµÄ³ĪĒåŹÆ»ŅĖ®²¢Õńµ“£¬Čō擵½³ĪĒåµÄŹÆ»ŅĖ®±ä»ė×Ē£¬ŌņŹĒCO2£»
£Ø3£©ČÜŅŗÖŠŅ»¶Øŗ¬ÓŠCaCl2£¬²»¹ÜŹĒ·ńÓŠĻ”ŃĪĖį£¬¶¼ÄÜŗĶAgNO3ČÜŅŗ·“Ӧɜ³É²»ČÜÓŚĻ”ĻõĖįµÄ°×É«³Įµķ£¬Ņņ“ĖøĆ·½°ø²»ĶźÉĘ£»
£Ø4£©¶žŃõ»ÆĢ¼ÄÜŗĶĒāŃõ»ÆÄĘ·“Ӧɜ³ÉĢ¼ĖįÄĘŗĶĖ®£¬Ņņ“Ė½«ÉŁĮæCO2ĘųĢåĶØČėŹ¢ÓŠ×ćĮæNaOHČÜŅŗµÄ¹ćæŚĘæÖŠ£¬ĘæÖŠČÜŅŗµÄÖŹĮæÓėÖ®Ē°Ļą±Č»įŌö¼Ó£»¹ŹŃ”A
æ¼µć£ŗ³£ÓĆĘųĢåµÄ·¢Éś×°ÖĆŗĶŹÕ¼Æ×°ÖĆÓėєȔ·½·Ø£»Ö¤Ć÷ŃĪĖįŗĶæÉČÜŠŌŃĪĖįŃĪ


 Äæ±ź²āŹŌĻµĮŠ“š°ø
Äæ±ź²āŹŌĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĶ¼ŹĒŹÕ¼ÆøÉŌļRĘųĢå²¢ÓĆĖ®¶ŌĖü½ųŠŠĪüŹÕ“¦ĄķµÄÕżČ·×°ÖĆ”£ÓÉĶ¼ÖŠŹµŃé×°ÖĆĶĘ²āøĆĘųĢåµÄÓŠ¹ŲŠŌÖŹ£¬ÕżČ·µÄŅ»×éŹĒ
| | A | B | C | D |
| ĆÜ¶Č£ØÓėæÕĘų±Č½Ļ£© | “óÓŚ | “óÓŚ | Š”ÓŚ | Š”ÓŚ |
| ŌŚĖ®ÖŠµÄČܽāŠŌ | ÄŃČÜ | ¼«Ņ×ČÜ | ¼«Ņ×ČÜ | ÄŃČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹŅĻÖÓŠĀČĖį¼Ų”¢“óĄķŹÆ”¢Ļ”ĮņĖį”¢Ļ”ŃĪĖį”¢¶žŃõ»ÆĆĢ¼°ĻĀĮŠŅĒĘ÷£ŗ
£Ø1£©Š“³öÉĻŹöŅĒĘ÷µÄĆū³Ę£ŗ¢Ł £¬¢Ū ”£
£Ø2£©ĄūÓĆÉĻŹöŅĒĘ÷¼°Ņ©Ę·ÖĘČ”ŗĶŹÕ¼ÆijÖÖĘųĢ壬ĖłŠčŅĒĘ÷ÓŠ £ØĢīŠņŗÅ£©£¬ÖĘČ”øĆĘųĢåµÄ»Æѧ·½³ĢŹ½ĪŖ ”£øĆĘųĢåµÄÓĆĶ¾ŹĒ
”££ØŠ“³öŅ»ÖÖ¼“æÉ£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
æÕĘųŹĒČĖĄą»ī¶Æ±ŲŠčµÄ×ŌȻ׏Ō“”£¹¤ŅµÉĻ³£ÓĆ·ÖĄėæÕĘųµÄ·½·ØÖĘČ”ŃõĘų£¬ŹµŃéŹŅ³£ÓĆĪļÖŹ·Ö½āµÄ·½·ØÖĘČ”ŃõĘų”£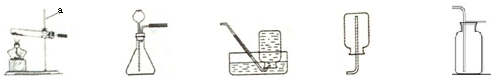
A B C D E
£Ø1£©Ķ¼ÖŠŅĒĘ÷aµÄĆū³ĘŹĒ ”£
£Ø2£©ŹµŃéŹŅÖĘČ”²¢ŹÕ¼ÆŃõĘų£¬æÉŃ”Ōń ×°ÖĆ£ØĢī×ÖÄø“śŗÅ£©£¬“Ė·ØÖĘČ”ŃõĘųµÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©¼ģ²éB×°ÖĆĘųĆÜŠŌµÄ·½·ØŹĒ£ØæɽčÖśĖ®ŗĶĶ¼ÖŠµÄĘäĖū×°ÖĆ£© ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
½įŗĻČēĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ»Ų“šÓŠ¹ŲĪŹĢā£®
¢ŁŠ“³ö×°ÖĆÖŠ±ąŗÅŅĒĘ÷µÄĆū³Ę£ŗa £¬b £»
¢ŚÓĆĀČĖį¼ŲÖĘČ”ŃõĘųæÉŃ”ÓƵķ¢Éś×°ÖĆĪŖ £¬æÉÓĆÅÅĖ®·ØŹÕ¼ÆŃõĘųµÄŌŅņŹĒ £®Š”Ć÷Ķ¬Ń§ÓĆÅÅĖ®·ØĪ“ÄÜŹÕ¼Æµ½Į½ĘæŃõĘų£¬ĘäæÉÄܵÄŌŅņÓŠ £ØĢīŠņŗÅ£©£»
A£®Ć»ÓŠČū½ōĻšĘ¤Čū
B£®Ć»ÓŠ¼°Ź±øü»»ŹÕ¼ÆµŚ¶žĘæ
C£®¼ÓČČĒ°ŅŃ½«¼ÆĘųĘæ¹ąĀśĖ®µ¹Į¢ÓŚĖ®²ŪÖŠ
¢Ū¼ģŃ鶞Ńõ»ÆĢ¼ŹĒ·ńŹÕ¼ÆĀśµÄ·½·ØŹĒ £»
¢ÜÓĆæéד“óĄķŹÆŗĶĻ”ŃĪĖįÖĘČ”¶žŃõ»ÆĢ¼Ź±£¬Ń”Ōń·¢Éś×°ÖĆDµÄÓŵćŹĒ £¬ŹµŃéŹŅ³£Ń”ŌńB×°Öƶų²»±ŲŃ”ŌńC×°ÖƵÄŌŅņŹĒ £¬×°ÖĆEŗĶ×°ÖĆ µÄŌĄķŹĒŅ»ŃłµÄ£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ŹµŃéŹŅÖĘČ”ĘųĢå³£³£ÓƵ½ĻĀĮŠ×°ÖĆ£¬øł¾Żøų³öµÄ×°ÖĆ»Ų“šĻĀĮŠĪŹĢā£ŗ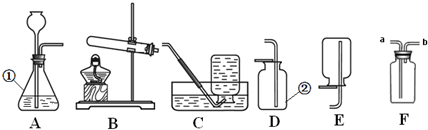
£Ø1£©Š“³ö±źŗÅŅĒĘ÷µÄĆū³Ę£ŗ¢Ł £»
£Ø2£©ČōøĆŹµŃéŃ”Ōń×°ÖĆAĄ“ÖĘČ”ŃõĘų£¬ĒėŠ“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£ÓĆC×°ÖĆŹÕ¼ÆŃõĘų£¬ÅŠ¶ĻŃõĘųŅŃŹÕ¼ÆĀśµÄĻÖĻóŹĒ ”£
£Ø3£©Ä³Ķ¬Ń§“ÓÉĻŹö×°ÖĆĶ¼ÖŠŃ”ÓĆŹŹµ±×°ÖĆ³É¹¦µÄÖʱøŗĶŹÕ¼ÆĮĖ¶žŃõ»ÆĢ¼£¬Ń”ÓƵÄ×°ÖĆŹĒ
£ØĢī×ÖÄø£©£®ĪŖĮĖ½ųŅ»²½ŃéÖ¤²śÉśµÄĘųĢåŹĒ¶žŃõ»ÆĢ¼£¬øĆĶ¬Ń§½«ĘųĢåĶØČėĶ¼F×°ÖĆÖŠ£¬ŌņF×°ÖĆÖŠÓ¦¼ÓČėµÄŹŌ¼ĮĪŖ £ØĢī»ÆѧŹ½£©£®
£Ø4£©ČōŅŖÖĘČ”ĒāĘų£¬ÓĆF×°ÖĆŹÕ¼Æ£¬ĘųĢåÓ¦“Ó £ØĢī”°a”±»ņ”°b”±£©µ¼¹ÜæŚĶØČė”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(8·Ö£©Ä³¶žŃõ»ÆĆĢѳʷ֊ŗ¬ÓŠŌÓÖŹĢ棬ĪŖ²ā¶ØøĆѳʷ֊¶žŃõ»ÆĆĢµÄÖŹĮæ·ÖŹż£¬Ä³ŠĖȤŠ”×éÉč¼ĘĮĖČēĻĀŹµŃé·½°ø£ŗŌŚŅ»¶ØĮæµÄѳʷ֊ĶØČėøÉŌļ“æ¾»µÄŃõĘų£¬Ź¹ŌÓÖŹĢæŌŚ¼ÓČČĢõ¼žĻĀ³ä·Ö·“Ӧɜ³ÉCO2Ą“½ųŠŠ·ÖĪö²ā¶Ø”£
£Ø1£©ŅĒĘ÷¢ŁµÄĆū³ĘŹĒ ”£Čē¹ūøĆŹµŃéŃ”ŌńĶ¼£ØŅ»£©×°ÖĆĄ“ÖĘČ”ŃõĘų£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø2£©ÓĆĶ¼£Ø¶ž£©×°ÖĆæÉÓĆÓŚŹÕ¼ÆŗĶøÉŌļŃõĘų£ŗČōÉÕĘæ³äĀśĖ®Ą“ŹÕ¼ÆŃõĘų£¬ĘųĢåÓ¦“Ó £ØĢī”°a”±»ņ”°b”±£¬ĻĀĶ¬£©¶ĖĶØČė£»ČōŌŚÉÕĘæČװČėÅØĮņĖį½ųŠŠøÉŌļĘųĢ壬ĘųĢåÓ¦“Ó ¶ĖĶØČė”£
£Ø3£©Ķ¼£ØČż£©ŹĒÓĆøÉŌļ“æ¾»µÄO2Óėѳʷ·“Ó¦Ą“²ā¶Ø¶žŃõ»ÆĆĢÖŹĮæ·ÖŹżµÄ×°ÖĆ£¬×°ÖĆ¢Ū֊װӊ¼īŹÆ»Ņ£ØÉśŹÆ»ŅÓė¹ĢĢåĒāŃõ»ÆÄʵĻģŗĻĪļ£©£¬Ęä×÷ÓĆŹĒ·ĄÖ¹æÕĘųÖŠ ½ųČė¢ŚÖŠ(ĢīŠ“»ÆѧŹ½)”£
£Ø4£©ĪŖŃéÖ¤Ķ¼£ØČż£©ÖŠ×°ÖĆ¢ŚŅŃ½«CO2ĪüŹÕĶźČ«£¬æÉŌŚ×°ÖĆ¢ŚÓė¢ŪÖ®¼ä¼ÓČėĶ¼£ØĖÄ£©×°ÖĆ½ųŠŠÖ¤Ć÷£¬ŌņĶ¼£ØĖÄ£©×°ÖĆÖŠ¼ÓČėµÄŹŌ¼ĮĪŖ £ØĢī×ÖÄø£©”£
A£®NaOHČÜŅŗ B£®³ĪĒåŹÆ»ŅĖ® C£®ÅØĮņĖį
£Ø5£©³ĘČ”5.0g¶žŃõ»ÆĆĢѳʷ½ųŠŠŹµŃ飬Ķ¼£ØČż£©ÖŠ×°ÖĆ¢Ś·“Ó¦Ē°ŗóµÄÖŹĮæ²īĪŖ1.1g£¬Ōņѳʷ֊¶žŃõ»ÆĆĢµÄÖŹĮæ·ÖŹżĪŖ £ØĢī¼ĘĖć½į¹ū£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø10·Ö£©øł¾ŻČēĻĀĶ¼ĖłŹ¾×°ÖĆ£¬½įŗĻĖłŃ§ÖŖŹ¶»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©Š“³öĶ¼ÖŠŅĒĘ÷¢ŁµÄĆū³Ę £»
£Ø2£©ŹµŃéŹŅÓĆA×°ÖĆÖĘČ”ŃõĘų£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½ £»
£Ø3£©ŹµŃéŹŅÖĘČ”¶žŃõ»ÆĢ¼Ó¦Ń”ÓƵķ¢Éś×°ÖĆŹĒ £¬ŅŖŠ“³ö·“µÄ»Æѧ·½³ĢŹ½£ŗ £»
£Ø4£©ŹµŃéŹŅÓĆB”¢E”¢C×°ÖĆÖĘČ”²¢ŹÕ¼ÆøÉŌļµÄŃõĘų£¬ŌņEÖŠÓ¦Ź¢·ÅµÄŹŌ¼ĮŹĒ £»
£Ø5£©ŹµŃéŹŅÓĆD×°ÖĆŹÕ¼ÆŃõĘų£¬µ± Ź±æŖŹ¼ŹÕ¼Æ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø5·Ö£©ĪŅĆĒѧĻ°ĮĖ³£¼ūĘųĢåµÄŹµŃéŹŅÖĘ·Ø£¬ÖŖµĄĮĖÖĘČ”ĘųĢåµÄŅ»°ćĖ¼Ā·ŗĶ·½·Ø”£
£Ø1£©ŹµŃéŹŅÖĘČ”ĘųĢåŹ±£¬Č·¶Ø·¢Éś×°ÖĆŠčŅŖæ¼ĀĒµÄŅņĖŲÓŠ £ØĢī×ÖÄøŠņŗÅ£©”£
| A£®·“Ó¦ĪļµÄדĢ¬ | B£®ĘųĢåµÄĆÜ¶Č |
| C£®·“Ó¦µÄĢõ¼ž | D£®ĘųĢåµÄČܽāŠŌ |

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com