
 ��ͼ��NaCl��MgSO4���ܽ�����ߣ���ش��������⣺
��ͼ��NaCl��MgSO4���ܽ�����ߣ���ش��������⣺

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
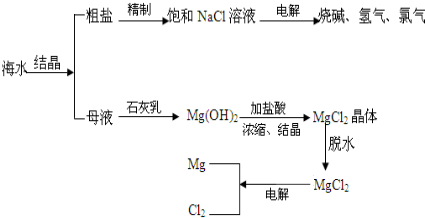
| ��Ʒ���ţ�Q/JSRF0001S ��֤�ţ�SD-013 ���ϱ�������ʳ���� ����� �����軯�� �Ȼ��ƣ�g/100g����98.00 ����⣨��I�ƣ�/��mg/kg��18-33 �����軯�أ���[Fe��CN��6]4-�ƣ���mg/kg����10.0��2����ȡ����þ����ȡ���κ�Ŀ�±�к��зḻMgCl2����Դ����ͼ�Ǵӿ�±����ȡþ�ļ����̣�  ���������У��Լ�Aͨ�������۵� Ca��OH��2 Ca��OH��2 ���ѧʽ������Һ������B��Mg��OH��2 Mg��OH��2 ���Լ�CΪHCl HCl ������ˮMgCl2��ȡMg�Ļ�ѧ����ʽΪMgCl2
MgCl2 ��
�鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ��2013?������һģ��ij���Σ���Ҫ�ɷ���NaCl����Ʒ�к�������ɳ����CaCl2��MgCl2���ʣ������dz�ȥ������Ʒ�����ʵ�ʵ�����̣����ݴ�����ͼ�ش� 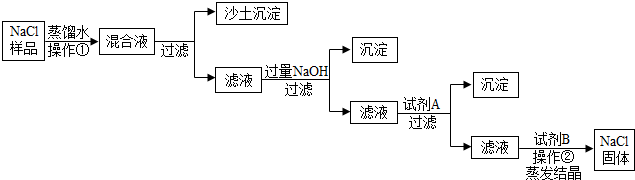 ��1�������ٵ������� �ܽ� �ܽ� ����2������NaOHĿ��������Һ��MgCl2��Ӧ�Ӷ���ȥ���ʣ�д��NaOH��MgCl2��Ӧ�Ļ�ѧ����ʽ 2NaOH+MgCl2=Mg��OH��2��+2NaCl 2NaOH+MgCl2=Mg��OH��2��+2NaCl ����3�������Լ�A��Ŀ��������Һ�е�CaCl2��Ӧ��������Լ�A������ CaCO3 CaCO3 �� ��4����ʵ���β��ù��˲�������ͼ�ǹ���װ�ã�����ʱ��Һ���ز�����������©�����㵹��ע��Һ��ʼ��Ҫ ������ֽ ������ֽ ��Ե����5�������������ᾧʱ�õ��IJ������������� ���裬��ֹ�ֲ��¶ȹ��ߣ����Һ�ηɽ� ���裬��ֹ�ֲ��¶ȹ��ߣ����Һ�ηɽ� ���鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ����� ��������Ҫ�Ļ���ԭ�� ��һ���Ʊ�̽������ͼ�ǹ�ҵ�����������Ҫ����ʾ��ͼ�� 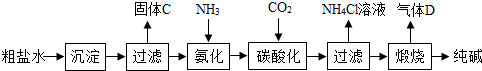 [��������] �ٴ���ˮ�к�������MgCl2��CaCl2�� �ڳ����£�NH3��������ˮ��CO2������ˮ�� ��NaHCO3�����ֽ⣬Na2CO3���Ȳ��ֽ⣮ ��1��д����ȥ����ˮ��MgCl2��CaCl2�Ļ�ѧ����ʽ�� MgCl2+2NaOH=Mg��OH��2��+2NaCl MgCl2+2NaOH=Mg��OH��2��+2NaCl ��CaCl2+Na2CO3=CaCO3��+2NaCl CaCl2+Na2CO3=CaCO3��+2NaCl ��2���ڹ�ҵ��������������У��ȡ���������̼�ữ����Ŀ���� ��������Һ����CO2���� ��������Һ����CO2���� ����̼�ữ��ʱ��NaCl��NH3��CO2 ��H2O���������NaHCO3��д���÷�Ӧ�Ļ�ѧ����ʽNaCl+NH3+CO2+H2O=NaHCO3+NH4Cl NaCl+NH3+CO2+H2O=NaHCO3+NH4Cl ����3����̼�ữ������˻�õ�NH4Cl������ �� �� �ʣ�Ҳ�������� ���� ������ȡ�����ȴ����NH4Cl��Һ���ټ�����ʯ�һ��ѭ��ʹ�õ�������NH3�������� NH3�������� ����4�������Ƶô���Ļ�ѧ����ʽ�� 2NaHCO3
2NaHCO3
�������ɷ�̽�� [�������]������Ʒ�к�����Щ���ʣ� [����]����һ�����ܺ���NaHCO3�� ����������ܺ���NaCl�� �������� NaHCO3��NaCl NaHCO3��NaCl [ʵ��̽��]ȷ���������Ƿ�NaHCO3��ʵ��װ�ú���Ҫʵ�鲽�����£� 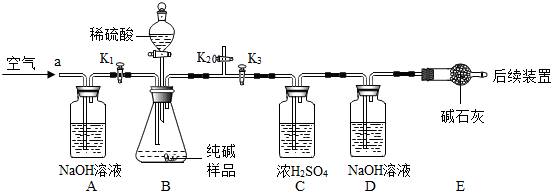 �ٳ���D��Eװ��������Ϊ200.0g����10.6 0g��������������ƿ�У�����ͼ��װ�����K1��K2���ر�K3����������һ��ʱ������� �ڹرջ���K1��K2����K3����������ϡ���ᣬ����ƿ�в��ٲ�������ʱ���ٴδ�ֹˮ��K1���ӵ���a���ٴλ������������ ��һ��ʱ����ٴγ���װ��D��E��������Ϊ204.84g�� [ʵ������] ��5��������Ʒǰ��Ӧ ���װ�������� ���װ�������� ����6��װ��A�������� ��ȥ�����еĶ�����̼ ��ȥ�����еĶ�����̼ ��װ��C����������ȥ������̼�е�ˮ ��ȥ������̼�е�ˮ ��װ��E�����������մ�D�д�����ˮ ���մ�D�д�����ˮ ����7����Ӧ�������ֹˮ��K1���������������Ŀ���� �����ɵĶ�����̼ȫ���͵�D�� �����ɵĶ�����̼ȫ���͵�D�� ��װ��B��һ�������Ļ�ѧ��Ӧ����ʽΪNa2CO3+H2SO4=Na2SO4+H2O+CO2�� Na2CO3+H2SO4=Na2SO4+H2O+CO2�� װ��D�еĻ�ѧ��Ӧ����ʽΪCO2+2NaOH=Na2CO3+H2O CO2+2NaOH=Na2CO3+H2O ����8��װ��B������CO2������Ϊ 4.84 4.84 g��ͨ������˵��������A A ��NaHCO3������ĸ����A��һ�� B��һ���� C������ D����ȷ�� ������̣���֪Na2CO3��Է�������Ϊ106��NaHCO3��Է�������Ϊ84���� ����10.6g��ƷȫΪ̼���ƣ����ɶ�����̼������Ϊx Na2CO3+H2SO4=Na2SO4+H2O+CO2�� 106 44 10.6g x
x=4.4g 4.4g��4.84g ������Ʒ�к���̼�����ƣ� ����10.6g��ƷȫΪ̼���ƣ����ɶ�����̼������Ϊx Na2CO3+H2SO4=Na2SO4+H2O+CO2�� 106 44 10.6g x
x=4.4g 4.4g��4.84g ������Ʒ�к���̼�����ƣ� ��9����ȡ10.6��Ʒ������a g 14.6%������ǡ����ȫ��Ӧ���ٽ�������Һ���ɺ�õ����������ΪW����W��ֵ���� W��0.32a W��0.32a ����ʱ����Ʒ�к���NaCl���鿴�𰸺ͽ���>> ��Ŀ�����л�ѧ ��Դ�� ���ͣ� ��ͼ��ij�ӵ�ʳ�ΰ�װ���ϵIJ������֣�
��1��20g��Ʒ�е���ص������� ��2����Ʒ�е�Ԫ�ص����������ж���Ʒ�е�Ԫ�صĺ����Ƿ���ϱ�ǩҪ�� �鿴�𰸺ͽ���>> ͬ����ϰ��� ����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר�� Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com��Ȩ��������վ�������£�ͼƬ��Դ�����磬����Ȩ����Ȩ��ԭ�������У�ת�������ַ���Ȩ��������Ȩ����������������֪�����ǽ����촦������ϵqq��3310059649�� ICP�������: ��ICP��07509807��-10 ����������42018502000812�� |