
£Ø4·Ö£©Õņ½ŹōÓŚ³ĒŹŠÉ½ĮÖ£¬ÓŠ×ÅÓĘ¾ĆµÄĪÄ»ÆŗĶĄśŹ·”£
£Ø1£©Ć©É½ŠĀĖľü¼ĶÄī¹ŻŹĒÕņ½ŹŠµÄĆūʬ֮Ņ»”£¹ŻÄŚÓŠŠķ¶ąæ¹Õ½Ź±µÄĒ¹ÅŚ£¬µ«ÓŠŠ©±ķĆę³öĻÖŠā¼££¬ĘäÖ÷ŅŖŌŅņŹĒ£ŗĢśÓė µČĪļÖŹ¹²Ķ¬×÷ÓĆµÄ½į¹ū”£ĖłŅŌ£¬Ę½Ź±Éś»īÖŠĪŅĆĒĪŖĮĖ·ĄÖ¹½šŹōÉśŠāæɲÉČ”µÄ“ėŹ©ŹĒ £ØŠ“³öŅ»ÖÖ£©”£
£Ø2£©”°ĪĄøŚĪĀČŖ”±ŹĒČĖĆĒŠŻĻŠµÄŗĆČ„“¦”£
¢ŁĪĀČŖĖ®µÄpHŌŚ7.5”«8.9Ö®¼ä£¬øĆĪĀČŖĖ®ĻŌ £ØĢī”°ĖįŠŌ”±”¢”°¼īŠŌ”±»ņ”°ÖŠŠŌ”±£©”£
¢Ś¼ģŃéøĆĪĀČŖĖ®ŹĒÓ²Ė®»¹ŹĒČķĖ®µÄ¼ņµ„·½·ØŹĒ ”£
£Ø1£©ŃõĘųŗĶĖ® ±£³Ö±ķĆę½ą¾»øÉŌļµČ£Ø“š°øŗĻĄķ¼“æÉ£©
£Ø2£©¢Ł¼īŠŌ ¢ŚČ”Ńł£¬¼Ó·ŹŌķĖ®£¬ÅŻÄ¶ąµÄĪŖČķĖ®”£
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ĢśÉśŠāŹĒĢśÓėŃõĘųŗĶĖ®Ķ¬Ź±½Ó“„£¬¹²Ķ¬×÷ÓĆµÄ½į¹ū£»Ę½Ź±Éś»īÖŠĪŖĮĖ·ĄÖ¹½šŹōÉśŠā£¬ĪŅĆĒæÉŅŌ×öµ½£ŗ±£³Ö±ķĆę½ą¾»øÉŌļ£¬ĶæÓĶµČ£Ø“š°øŗĻĄķ¼“æÉ£© £»£Ø2£©¢ŁČÜŅŗpH=7ĻŌÖŠŠŌ£¬“óÓŚ7ĻŌ¼īŠŌ£¬Š”ÓŚ7ĻŌĖįŠŌ£¬ĪĀČŖĖ®µÄpHŌŚ7.5”«8.9Ö®¼ä “óÓŚ7£¬ĻŌ¼īŠŌ ¢Ś¼ų±šÓ²Ė®ŗĶČķĖ®µÄ¼ņµ„·½·ØŹĒ¼Ó·ŹŌķĖ®£¬ÅŻÄ¶ąµÄĪŖČķĖ®£¬ÅŻÄÉŁµÄĪŖÓ²Ė®.
æ¼µć£ŗøÖĢśµÄÉśŠāÓė·ĄŠā£»Ó²Ė®ÓėČķĖ®µÄ¼ģŃ飻ČÜŅŗĖį¼īŠŌÓėpHµÄ“󊔹ŲĻµ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ēė»Ų“šĻĀĮŠø÷ĪŹ£ŗ
£Ø1£©µĒɽ”¢Ē±Ė®”¢Ņ½ĮĘ¼±¾ČŹ±¶¼ŠčŅŖÓĆŃõĘų£¬ŹĒŅņĪŖŃõĘų ”£
£Ø2£©ŹŹĮæÉćČėĪ¢ĮæŌŖĖŲ¶ŌČĖĢå½”æµŗÜÖŲŅŖ£¬ČĖĢåČōȱɣ ŅײśÉśČ£³Ż”£
£Ø3£©ÓĆ»Æѧ·½³ĢŹ½»Ų“š£ŗ
Ļ”ĮņĖį³żĢśŠā£ŗ £»
²»ŌŚČÜŅŗÖŠ·¢ÉśµÄÖĆ»»·“Ó¦£ŗ ”£
£Ø4£©Ņ°“¶Ź±£¬ČĖĆĒ³£°Ń²ń²Ż¼ÜæÕ£¬Ź¹Č¼ÉÕøüĶś£¬ĒėĖµĆ÷ĘäÖŠµÄµĄĄķ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø3·Ö£©2014Äź3ŌĀ22ČÕŹĒµŚ¶žŹ®¶ž½ģ”°ŹĄ½ēĖ®ČÕ”±£¬3ŌĀ22”Ŗ28 ČÕŹĒµŚ¶žŹ®Ęß½ģ”°ÖŠ¹śĖ®ÖÜ”±”£ĮŖŗĻ¹śČ·¶Ø2014Äź”°ŹĄ½ēĖ®ČÕ”±µÄŠū“«Ö÷ĢāŹĒ”°Ė®ÓėÄÜŌ“£ØWater and Energy£©”±”£ĪŅ¹ś¼ĶÄī2014Äź”°ŹĄ½ēĖ®ČÕ”±ŗĶ”°ÖŠ¹śĖ®ÖÜ”±»ī¶ÆµÄŠū“«Ö÷ĢāĪŖ”°¼ÓĒæŗÓŗž¹ÜĄķ£¬½ØÉčĖ®ÉśĢ¬ĪÄĆ÷”±”£Ä³Š£»·±£Š”×éµÄĶ¬Ń§ĻģÓ¦ÕāŅ»Ö÷Ģā£¬¶ŌćėŗÓŗÓĖ®½ųŠŠ¼ģ²ā”£
£Ø1£©½ü¼øÄźćėŗÓµÄŗÓĖ®Ō½·¢»ė×Ē”£³żČ„Ė®ÖŠÄŃČÜŠŌŌÓÖŹ½ųŠŠµÄ²Ł×÷ŹĒ £ØĢī×ÖÄø£©”£
A£®¹żĀĖ B£®½į¾§ C£®Õō·¢
£Ø2£©ĪŖĮĖ±£»¤ćėŗÓµÄĖ®ÖŹ£¬ĻĀĮŠ×ö·ØŗĻĄķµÄŹĒ (Ģī×ÖÄø)”£
A£®½ūÓĆÅ©Ņ©ŗĶ»Æ·Ź
B£®ŅÖÖĘĖ®ÖŠĖłÓŠ¶Æ”¢Ö²ĪļÉś³¤
C£®ČĪŅāÅÅ·ÅÉś»īĪŪĖ®
D£®¹¤Ņµ·ĻĖ®¾¹ż¾»»ÆŗóŌŁÅÅ·Å
£Ø3£©ŌŚČÕ³£Éś»īÖŠ£¬Čē¹ūĖ®µÄÓ²¶Č¹ż“ó»ņÕß²”ŌĪ¢ÉśĪļ¹ż¶ą£¬¶¼æÉŅŌÓĆ µÄ·½·ØĄ“½µµĶĖ®µÄÓ²¶ČŗĶɱƚ²”ŌĪ¢ÉśĪļ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ČēĶ¼ŹĒ²ā¶ØæÕĘųÖŠŃõĘųĢå»ż·ÖŹżµÄŹµŃé×°ÖĆ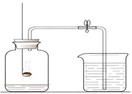
Ēė»Ų“šŅŌĻĀÓŠ¹ŲĪŹĢā£ŗ
£Ø1£©Č¼ÉÕ³×ÄŚŹ¢·ÅµÄĪļÖŹŹĒ £¬ÄÜ·ńÖ±½ÓøÄÓĆľĢ攢Įņ»ĒµČĪļÖŹ×ö“ĖŹµŃé£æŹŌĖµĆ÷ĄķÓÉ ”£
£Ø2£©øĆŹµŃéĖłÄܹŪ²ģµ½µÄĻÖĻóĪŖ £¬øł¾ŻøĆŹµŃéµÄŌĄķ£¬ÓŠĶ¬Ń§ČĻĪŖÖ»ŅŖ¶ŌøĆŹµŃé½ųŠŠŹŹµ±øĽų£¬ÓĆľĢ攢Įņ»Ē¾łæÉ“ļµ½Ō¤ĘŚÄæµÄ£¬ĒėÄćÅŠ¶ĻøĆĶ¬Ń§æÉÄÜ×öŌõŃłµÄøĽų£æ £¬æÉÄÜ·¢ÉśµÄ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ė®ŌŚ»ÆѧŹµŃéÖŠµÄ×÷ÓĆ²»æÉŗöŹÓ”£ČēĶ¼ĖłŹ¾µÄĖÄøöŹµŃéÖŠ·Ö±šÓƵ½Ė®”£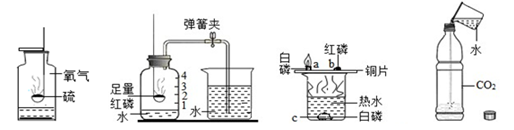
| A£®ĮņŌŚŃõĘųÖŠČ¼ÉÕ | B£®²ā¶ØæÕĘųÖŠŃõĘųŗ¬Įæ | C£®Ģ½¾æČ¼ÉÕĢõ¼ž | D£®Ģ½¾æ¶žŃõ»ÆĢ¼ŠŌÖŹ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø13·Ö£©2014Äź3ŌĀ22ČÕŹĒµŚ22½ģ”°ŹĄ½ēĖ®ČÕ”±£¬ĒėÄćøł¾ŻĖłŃ§£¬»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©ĻĀĶ¼ŹĒ¼ņŅ×¾»Ė®×°ÖĆ”£øĆ¾»Ė®×°ÖĆ £ØĢī”°ÄÜ”±»ņ”°²»ÄÜ”±£©½«ŗÓĖ®±ä³É“æĖ®”£
£Ø2£©¶žŃõ»ÆĀČ£ØClO2£©ŹĒŠĀŅ»“śŅūÓĆĖ®µÄĻū¶¾¼Į£¬¹¤ŅµÉĻ½«ĀČĘų£ØCl2£©ĶØČėŃĒĀČĖįÄĘ£ØNaClO2£©ČÜŅŗÖŠ·“Ó¦Ą“ÖĘČ”¶žŃõ»ÆĀČ£¬Ķ¬Ź±Éś³ÉĀČ»ÆÄĘ”£Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ ”£
£Ø3£©Š”ĄöŹÕ¼Æµ½Ņ»Ęæ»ė×ĒµÄŗÓĖ®£¬ĖżŅŖÄ£Äā×ŌĄ“Ė®³§µÄ¾»Ė®¹ż³Ģ£¬×īÖÕÖĘ³ÉÕōĮóĖ®£¬
¹ż³ĢČēĻĀĶ¼ĖłŹ¾”£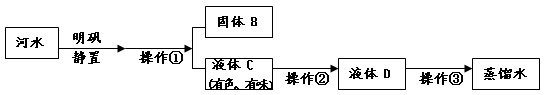
²Ł×÷¢ŁµÄĆū³ĘŹĒ £»²Ł×÷¢ŚÓ¦Ń”ÓƵÄ×°ÖĆŹĒĻĀĶ¼ÖŠµÄ £ØĢīŠņŗÅ£¬ĻĀĶ¬£©£»²Ł×÷¢ŪӦєÓƵÄ×°ÖĆŹĒĻĀĶ¼ÖŠµÄ ”£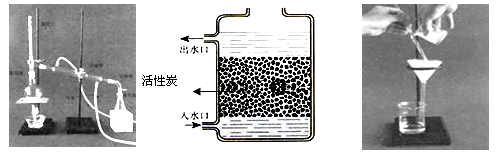
£Ø¢ń£© £Ø¢ņ£© £Ø¢ó£©
£Ø4£©Š”ĄöȔɣĮæŅŗĢåDÓŚŹŌ¹ÜÖŠ£¬¼ÓČėÉŁĮæ·ŹŌķĖ®£¬Õńµ“£¬·¢ĻÖÓŠ½Ļ¶ąø”Ōü²śÉś£¬ĖµĆ÷ŅŗĢåDŹĒ Ė®”££ØĢī”°Ó²”±»ņ”°Čķ”±£©
£Ø5£©ČēĶ¼ŹĒ¼×”¢ŅŅĮ½ÖÖĪļÖŹµÄČܽā¶ČĒśĻߣ¬»Ų“šĻĀĮŠĪŹĢā£ŗ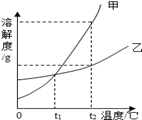
¢Ł________”ꏱ£¬¼×”¢ŅŅĮ½ÖÖĪļÖŹµÄČܽā¶ČĻąµČ£»
¢ŚČō¹ĢĢå¼×ÖŠŗ¬ÓŠÉŁĮæŅŅŹ±æÉÓĆ_____________·½·ØĢį“æ¼×£ØĢī”±Õō·¢½į¾§”°»ņ”±ĄäČ“½į¾§”°£©£»
¢Ūt2”ęÓƵČÖŹĮæµÄ¼×”¢ŅŅĮ½ÖÖĪļÖŹÅäÖĘ¼×”¢ŅŅµÄ±„ŗĶČÜŅŗŠčŅŖĖ®µÄÖŹĮæ:
¼×______ŅŅ£ØĢī”°£¾”±”°£¼”±»ņ”°=”±£©£»
¢ÜŹµŃéŹŅŠčŅŖÅäÖĘ50gČÜÖŹÖŹĮæ·ÖŹżĪŖ4%µÄĒāŃõ»ÆÄĘČÜŅŗ£¬ĖłŠč¹ĢĢåĒāŃõ»ÆÄʵÄÖŹĮæĪŖ___ __g£¬Ö÷ŅŖ²Ł×÷²½ÖčŹĒ£ŗ¼ĘĖć”¢_______”¢ĮæČ””¢Čܽā£®ŌŚČܽā¹ż³ĢÖŠÓĆ²£Į§°ō½Į°č£¬Ęä×÷ÓĆŹĒ______ ___£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
(6·Ö)Ė®ŹĒ×īĘÕĶØ”¢×ī³£¼ūµÄĪļÖŹÖ®Ņ»”£
£Ø1£©³¤ĘŚŹĒŅūÓĆÓ²Ė®²»ĄūÓŚ½”æµ£¬Ó²Ė®ŗĶČķĖ®µÄĒų±šŹĒ_______________________”£
£Ø2£©µē½āĖ®æÉÖ¤Ć÷Ė®ÓÉĒā”¢ŃõĮ½ÖÖŌŖĖŲ×é³É£¬øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø3£©Ė®ŹĒÖŲŅŖµÄČܼĮŗĶ»Æ¹¤ŌĮĻ”£ĀČ¼ī¹¤ŅµŅŌ±„ŗĶŹ³ŃĪĖ®ĪŖŌĮĻ»ńµĆÉÕ¼īµČ»Æ¹¤²śĘ·£¬·“Ó¦ŌĄķĪŖ:2NaCl+2H2OĶصē2NaOH+H2”ü+Cl2”ü”£
¢Ł 20”ꏱ£¬NaClµÄČܽā¶ČŹĒ36g”£øĆĪĀ¶ČĻĀ£¬±„ŗĶŹ³ŃĪĖ®ÖŠČÜÖŹÓėČܼĮµÄÖŹĮæ±ČĪŖ ”£
¢Ś ÉÕ¼īæÉÓĆÓŚ“¦ĄķĮņĖįŠ¹Ā©£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
£Ø4£©Ė®ŌŚ»ÆѧŹµŃéÖŠ¾ßÓŠÖŲŅŖ×÷ÓĆ”£½«ĢśĖæ·ÅŌŚ
³±ŹŖµÄæÕĘųÖŠ£ØČēĶ¼ĖłŹ¾£©£¬Ņ»¶ĪŹ±¼äŗó£¬
¹Ū²ģµ½µ¼¹ÜÄŚŅŗĆęÉĻÉż;“ņæŖK£¬µĪ¼ÓĻ”ŃĪĖį£¬
¹Ū²ģµ½µÄĻÖĻóŹĒ ”£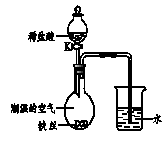
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
“æ¾»ĪļµÄ»ÆѧŹ½ŹĒ¾ŹµŃé²ā¶ØµĆ³öµÄ”£Ķ¬Ń§ĆĒÉč¼ĘŅŌĻĀ¼×”¢ŅŅĮ½×鏵Ńé·½°ø²ā¶ØĖ®µÄ×é³É”£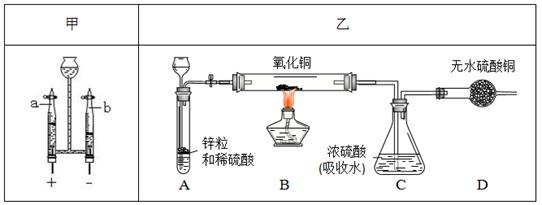
£Ø1£©¼×·½°øÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £¬b¶ĖµÄĘųĢåŹĒ ”£ÓɲśÉśĘųĢåµÄĢå»żæÉĶĘĖć³öĒāĘųÓėŃõĘųµÄÖŹĮæ±ČĪŖ1£ŗ8£¬ČōĒā”¢ŃõŌŖĖŲµÄĻą¶ŌŌ×ÓÖŹĮæ·Ö±šĪŖmŗĶn£¬ŌņĖ®ÖŠĒā”¢ŃõŌŖĖŲµÄŌ×ÓøöŹż±ČĪŖ £ØÓĆ”°m”±»ņ”°n”±±ķŹ¾£©”£
£Ø2£©ŅŅ·½°øµÄŹµŃé¹ż³ĢÖŠ£¬B×°ÖĆÖŠæɹŪ²ģµ½ ”£Čō·“Ó¦½įŹų²āµĆB×°ÖĆ¼õĒį1.6g£¬ŌņC×°ÖĆŌöÖŲµÄÖŹĮæ»į (Ģī”°“óÓŚ”±”¢”°µČÓŚ”±»ņ”°Š”ÓŚ”±)1.8g”£¼×”¢ŅŅ·½°øµÄ·“Ó¦ŌĄķĖä²»Ķ¬£¬µ«ŹµŃéµÄÉč¼ĘŅĄ¾Ż¶¼ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
Ė®ŹĒČĖ¼°Ņ»ĒŠÉśĪļÉś“ęĖł±ŲŠčµÄ£¬ĪŖĮĖČĖĄąŗĶÉē»į¾¼ĆµÄæɳ֊ų·¢Õ¹£¬ĪŅĆĒÓ¦øĆĮĖ½āŅ»Š©ÓŠ¹ŲĖ®µÄÖŖŹ¶”£ĒėÄć»Ų“š£ŗ
£Ø1£©ĢģČ»Ė®ÖŠŗ¬ÓŠŠķ¶ąŌÓÖŹ£¬æÉĄūÓĆĪüø½”¢³Įµķ”¢¹żĀĖŗĶÕōĮóµČ·½·Ø¾»»Æ£¬ĘäÖŠ¾»»ÆĖ®³Ģ¶Č×īøߵķ½·ØŹĒ £»³żČ„Ė®ÖŠÄŃČÜŠŌ¹ĢĢåŌÓÖŹµÄ²Ł×÷ŹĒ £¬“Ė²Ł×÷ÖŠŅŖÓƵ½²£Į§°ō£¬Ęä×÷ÓĆŹĒ ”£
£Ø2£©Éś»īÖŠæÉŅŌ²ÉÓĆ Ēų·ÖÓ²Ė®ŗĶČķĖ®£¬æÉŅŌĶعż¼ÓČė Ą“Īüø½Ė®ÖŠÓŠŅģĪ¶µÄĪļÖŹ”£
£Ø3£©ČēĶ¼ŹĒµē½āĖ®ŹµŃé×°ÖĆ”£ĻĀĮŠ¹ŲÓŚµē½āĖ®ŹµŃéµÄŠšŹöÖŠ£¬ÕżČ·µÄŹĒ £ØĢīŠņŗÅ£©”£
| A£®øĆŹµŃéæÉÖ¤Ć÷Ė®ÓÉĒāŌŖĖŲŗĶŃõŌŖĖŲ×é³É |
| B£®ŹŌ¹ÜaÖŠŹÕ¼ÆµÄĘųĢåŹĒŃõĘų |
| C£®ŹŌ¹ÜaŗĶŹŌ¹ÜbÖŠŹÕ¼ÆµÄĮ½ÖÖĘųĢåµÄÖŹĮæ±ČŹĒ2:1 |
| D£®ŌŚĖ®ÖŠ¼ÓÉŁĮæĻ”ĮņĖįæÉŌöĒæĖ®µÄµ¼µēŠŌ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com