
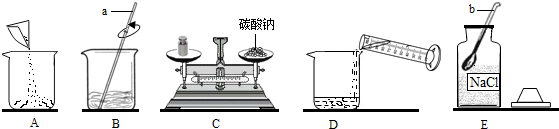
���� ��1����dz������������ƣ�
��2������������Һ�IJ��迼�ǣ�
��3�����������������ʱ�������㷨���ǣ�
��4������̼���Ƶ�����Կ��ǣ�
��5��������pH��ֽ�ⶨ��������Һ��pHֵ�ķ������ǣ�
��� �⣺��1��ͨ������������ָ���������ÿ�֪��a�Dz�������b��ҩ�ף�
��2������������������һ������Һ�Ļ������裺���㡢��������ȡ���ܽ⡢װƿ��ţ�����ʵ�����ȷ����˳��Ϊ��ECADB��
��3�������������ʱ����=���������-���������Ϊ��10g-3.5g=6.5g��
��4������̼�����Լ���pHֵ����7����ˮʪ�����Ϊˮ��pHֵ����7�����Խ����ƫС��
��5����pH��ֽ�ⶨ��������Һ��pHֵ�ķ����ǣ��ڰ״ɰ����Ƭ�Ϸ�һСƬpH��ֽ���ýྻ�IJ�����պȡ����Һ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�����Һ��pHֵ��
�ʴ�Ϊ����1����������ҩ�ף�
��2��ECADB��
��3��6.5��
��4��ƫС��
��5���ڰ״ɰ����Ƭ�Ϸ�һСƬpH��ֽ���ýྻ�IJ�����պȡ����Һ�ε���ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ����ɵó�����Һ��pH��
���� �����ؼ���Ҫ֪��������Һ�IJ��裬��ƽ�������������ļ��㷽������ˮ��ʪ��pHֵ�ı仯����pHֵ�ľ��巽����


 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �٢ۢ� | D�� | �٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ԭ�ӵ�������ƽ��������ԭ�Ӻ��� | |
| B�� | ԭ��ͨ����ʧ�����γ����ӣ������Ӳ����γ�ԭ�� | |
| C�� | ԭ�ӡ����ӡ����ӡ����Ӷ��ǹ������ʵ����� | |
| D�� | �ɷ��ӹ��ɵ����ʷ�����ѧ�仯ʱ�����ӱ����ı� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����������ȼ�գ���������ɫ�Ļ��� | |
| B�� | ������������ȼ�գ����������İ��� | |
| C�� | ̼�ڿ�����ȼ�գ�����ҫ�۵ĺ�⣬���ɺ�ɫ���� | |
| D�� | ��˿��������ȼ�գ��������䣬���ɺ�ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
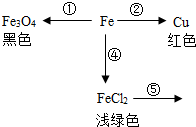 ��ѧʹ������Ѥ����ʣ���ͼ��������������֮��Ĺ�ϵ����ɫ�仯����д����
��ѧʹ������Ѥ����ʣ���ͼ��������������֮��Ĺ�ϵ����ɫ�仯����д�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ˮ�Ǿ����̶Ƚϸߵ�ˮ | B�� | ����ˮ�ɼ���Ӳˮ����ˮ | ||
| C�� | ����̿�ɽ���ˮ��Ӳ�� | D�� | ���˿ɳ�ȥˮ�в����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | -1 | B�� | +1 | C�� | +3 | D�� | +4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��һ���ȶ���Һ�嶼����Һ | |
| B�� | ��Һϡ��ǰ����Һ���������� | |
| C�� | ��Һ��ֻ��һ������ʱ����ҺΪ������ | |
| D�� | �������ܽ�õ���Һ�Ĺ����У�ͨ���з��Ȼ����ȵ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com