
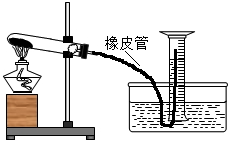
 ��2013?���ݣ���ͼ��ʾ��Ϊ�˲ⶨʵ�����и��������Ʒ�Ĵ��ȣ��������ʲ��μӷ�Ӧ����С���ȡ4.0g����Ʒ���Թ��м��ȣ�����Ͳ�ռ����ɵ����壮���������ȫ�ֽ����ȥ�ƾ��ƣ���װ����ȴ�����ºõ�0.23���������������������ܶ�ԼΪ1.4��/������
��2013?���ݣ���ͼ��ʾ��Ϊ�˲ⶨʵ�����и��������Ʒ�Ĵ��ȣ��������ʲ��μӷ�Ӧ����С���ȡ4.0g����Ʒ���Թ��м��ȣ�����Ͳ�ռ����ɵ����壮���������ȫ�ֽ����ȥ�ƾ��ƣ���װ����ȴ�����ºõ�0.23���������������������ܶ�ԼΪ1.4��/������
| ||
| 316 |
| 32 |
| X |
| 0.32�� |
| 3.16�� |
| 4.0�� |


 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
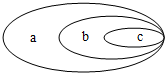 ��2013?���ݣ���ͼ��ʾa��b��c��������֮��Ĺ�ϵ������ѡ���и�����֮�䲻�������ֹ�ϵ���ǣ�������
��2013?���ݣ���ͼ��ʾa��b��c��������֮��Ĺ�ϵ������ѡ���и�����֮�䲻�������ֹ�ϵ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
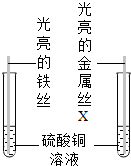 ��2013?���ݣ��ϴ�ͬѧΪ�˱Ƚ�����ͭ��δ֪����X�Ļ�Դ�С���������ͼ��ʾʵ�飮
��2013?���ݣ��ϴ�ͬѧΪ�˱Ƚ�����ͭ��δ֪����X�Ļ�Դ�С���������ͼ��ʾʵ�飮�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com