��2008?�人��ij�������̼���ƺ�����������ɣ�ʵ������������ͼ��ʾװ�ø�һ�ף�װ�������Ծ����ã�ijͬѧ������װ��װ�ã�ʵ��ⶨ�û������̼���Ƶ�������������֪��ʯ��Ϊ���������������ƵĻ����������շ�Ӧ���ɵ��������ʣ���Ӧǰ��������������ֱ�Ϊm1��m2��ʵ��ǰ�������١��ھ����ڹر�״̬������ݸ�ͬѧ��ʵ������ش��й����⣮
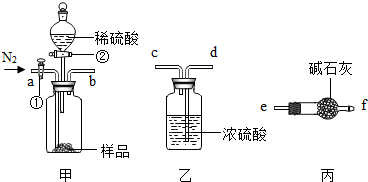
��1����ͼ��ʾ��ȡһ������Ʒ������У�Ϊ��֤ʵ������ȷ�ԣ�������װ�ýӿ�b-
d
d
��ѡ����ĸc��d��e�����ٴ٣�����ͨ�뵪��һ��ʱ���رբ٣���ʱͨ��������Ŀ����
��װ���еĿ�����������У�����ʵ�����
��װ���еĿ�����������У�����ʵ�����
��
��2����������ʣ��װ�ú�ڣ�������������ϡ�����رբڣ������в��������ݲ���ʱ���ٴ٣�����ͨ�뵪������ʱͨ�뵪����Ŀ����
��������װ���еĶ�����̼����ȫ��������У�����ʵ�����
��������װ���еĶ�����̼����ȫ��������У�����ʵ�����
��
��3����ʵ���У�װ���ҵ�������
����ˮ
����ˮ
��
��4������������װ����ԭ����Ҳ�ܲ���û������̼���Ƶ��������������һ�����û�ѧ����ʽ��ʾ��
Na2CO3+Ca��OH��2�TCaC03��+2NaOH��
Na2CO3+Ca��OH��2�TCaC03��+2NaOH��
��



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
