
 С��ͬѧΪ�˲ⶨ�������е�̼��Ƶ����������������������飺��������ϴ�������ﲢ����������ƿ�����������ϡ�����ַ�Ӧ�������������ʲ������ᷴӦ����
С��ͬѧΪ�˲ⶨ�������е�̼��Ƶ����������������������飺��������ϴ�������ﲢ����������ƿ�����������ϡ�����ַ�Ӧ�������������ʲ������ᷴӦ�������� �����ݴ�����
��1������̼�����ϡ���ᷴӦ�ų��˶�����̼���壬��Ӧ��ʣ������������ȷ�Ӧǰ�ļ��٣����������غ㶨��֪�����ٵ������������ɶ�����̼��������
��2���ɶ�����̼����������̼��������ᷴӦ�Ļ�ѧ����ʽ���Լ������������̼��Ƶ�����������
����˼�����ۡ����ݼ�����̷����жϣ�
��� �⣺�����ݴ�����
��1���������غ㶨�ɿ�֪�����ɵĶ�����̼�����ǣ�12.5g+85.5g-93.6g=4.4g��
��2���輦������̼��Ƶ�����Ϊx��
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 4.4g
$\frac{100}{44}=\frac{x}{4.4g}$ ��ã�x=10g
��������̼��Ƶ���������Ϊ��$\frac{10g}{12.5g}$��100%=80%
����˼�����ۡ�
ʵ���������в������ɵĶ�����̼�������ܽ���ˮ��û��ȫ���ݳ�����ɼ�������ʵ��ֵ���ƫС��
�ʴ�Ϊ�������ݴ�������1��4.4g����2����������̼��Ƶ���������80%������˼�����ۡ�ƫС��
���� �����Ĺؼ���Ҫ֪�����������ļ������������ɶ�����̼������������������������̼��ƺ��Ȼ��Ƶ��������ټ��������������ɣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��+�������������+���� | B�� | ��+������������ | ||
| C�� | ������ء������+��������+���� | D�� | ʯ��+������������̼+ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ��������Ŀ��� | |
| B�� | Һ��������������ȫ�ֽ��ʣ��Һ�� | |
| C�� | �����������ơ�� | |
| D�� | ��ˮ����������ؼ��Ⱥ�Ĺ���ʣ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯī���� | B�� | ����̿����ˮ | ||
| C�� | �õ����� | D�� | ��ʯīת���ɽ��ʯ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ˮ������ | B�� | ��ˮͨ������õ���ˮ | ||
| C�� | ˮ�������ˮ���� | D�� | ����Һ̬����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
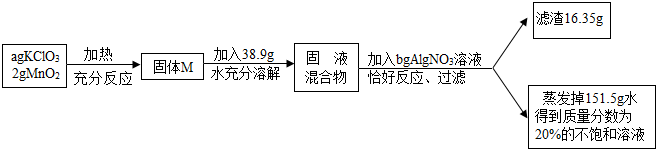
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 6.0g����ȫ����O2 | |
| B�� | 6.0g����ͨ������NaOH��Һ���������4.4g | |
| C�� | ��Ӧ��Ĺ����ǻ���� | |
| D�� | ����Na2CO3������Ϊ15.9g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com