£Ø2012?Ī«·»¶žÄ££©¢ń£®“ŗÅÆ»ØæŖ£¬ĄĻŹ¦×éÖÆĶ¬Ń§ĆĒµ½½¼ĶāŅ°“¶£®»ī¶ÆÖŠ£¬Ķ¬Ń§ĆĒŠÆ“ųĮĖĢś¹ų”¢¼¦µ°”¢ĆęĢõ”¢Ī÷ŗģŹĮ”¢Ź³ÓĆÓĶ”¢Ź³ŃĪ”¢Ī¶¾«”¢Ź³“×”¢Č„ĪŪ·Ū£ØÓŠŠ§³É·ŻĪŖĢ¼ĖįÄĘ£©µČĪļĘ·£®
£Ø1£©“ÓĶ¬Ń§ĆĒĖł“ųµÄŹ³ĪļÖŠĢōŃ”³öÄÜĪŖČĖĢåĢį¹©½Ļ¶ąĪ¬ÉśĖŲµÄŅ»ÖÖŹ³ĪļŹĒ
Ī÷ŗģŹĮ
Ī÷ŗģŹĮ
£ØĢīŠ“ĖłŃ”Ź³ĪļµÄĆū³Ę£©£®
£Ø2£©ŠÆ“ų¹ż³ĢÖŠ£¬²»É÷½«×°Ź³ŃĪ”¢Č„ĪŪ·ŪµÄĘæ×Ó»ģĻżĮĖ£®ÓŠµÄĶ¬Ń§½ØŅ飬ÓĆĖł“ųĪļĘ·ÖŠµÄ
Ź³“×
Ź³“×
£ØĢīĖłŠÆ“ųµÄĪļĘ·Ćū³Ę£©¾ĶæÉŅŌ½«ĖüĆĒĒų·ÖæŖĄ“£®
£Ø3£©ŌŚ”°Ōī”±ÉĻŠü¹ŅŅ°“¶¹ųŹ±£¬Š”Ć÷Éś»šŗ󊔊ĵ÷½ŚŅ°“¶¹ųµ½ŗĻŹŹµÄøß¶Č£®ÕāŃł×öµÄÖ÷ŅŖŌŅņŹĒ
C
C
£®
A£®±ÜĆāĢś¹ų±»æ¾»µ B£®±ÜĆāŹ³Īļæ¾ŗżĮĖ C£®Ź¹ÓĆĶāŃę¼ÓČČ
£Ø4£©ÖóĢĄŹ±£¬ĪŖĮĖ½āĢĄµÄĻĢĪ¶ŹĒ·ńŹŹŅĖ£¬Š”“ŗĶ¬Ń§Č”¹ųÖŠÉŁĮæĢĄĘ·³¢£®Č”ÉŁĮæĢĄ½ųŠŠĘ·³¢¾ĶæÉÖŖµĄÕū¹ųĢĄĻĢĪ¶ČēŗĪ£¬ŹĒŅņĪŖČÜŅŗ¾ßÓŠ
¾łŅ»ŠŌ
¾łŅ»ŠŌ
£ØĢīŠ“Ņ»ĢõŠŌÖŹ£©£®
£Ø5£©Ņ°“¶½įŹųŹ±£¬Š”»ŌÓĆøɲ¼ÄØČ„Ģś¹ų±ķĆęµÄĖ®Ö飮ÕāŃł×öµÄÄæµÄŹĒ
·ĄÖ¹ÉśŠā
·ĄÖ¹ÉśŠā
£®
¢ņ£®ĪŖ»ŗ½āÄÜŌ“½ōÕÅדæö£¬¹ś¼ŅŅ³ŃŅĘųŹ®¶žĪå¹ę»®³öĢØ£®
£Ø1£©Ņ³ŃŅĘųÓėĢģČ»ĘųµÄÖ÷ŅŖ³É·Ż¶¼ŹĒ¼×Ķ飬¼×ĶéŌŚæÕĘųÖŠĶźČ«Č¼ÉյĻÆѧ·½³ĢŹ½ĪŖ
£®ĢģČ»ĘųÓėĆŗ”¢
ŹÆÓĶ
ŹÆÓĶ
ŗĻ³ĘĪŖČż“ó»ÆŹÆČ¼ĮĻ£®
£Ø2£©Ņ³ŃŅĘųæŖ·¢½«øıäĪŅ¹śĻÖŌŚµÄÄÜŌ“øń¾Ö£¬ĪŖ»·¾³±£»¤“ųĄ“»ż¼«Ó°Ļģ£®ĻĀĮŠ»·¾³ĪŹĢāÓėČ¼Ćŗƻӊֱ½Ó¹ŲĻµµÄŹĒ
C
C
£ØĢī×ÖÄø£©£®
A£®ĖįÓź B£®ĪĀŹŅŠ§Ó¦ C£®³ōŃõ²ćĘĘ»µ D£®æÉĪüČėæÅĮ£ĪļŌö¼Ó
£Ø3£©Ęū³µĪ²ĘųŹĒ“óĘųĪŪČ¾Ö÷ŅŖĄ“Ō“Ö®Ņ»£®”°ČżŠ§“ß»Æ×Ŗ»»Ę÷”±æɽ«Ęū³µĪ²ĘųÖŠÓŠ¶¾ĘųĢ哦ĄķĪŖĪŽ¶¾ĘųĢ壬ĻĀĶ¼ĪŖøĆ·“Ó¦µÄĪ¢¹ŪŹ¾ŅāĶ¼£® »Ų“šĻĀĮŠĪŹĢā£®
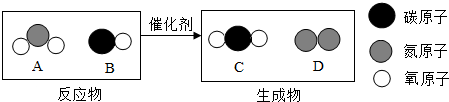
¢ŁAÖŠĮ½ÖÖŌŖĖŲÖŹĮæ±ČĪŖ
7£ŗ16
7£ŗ16
£®
¢Ś4ÖÖĪļÖŹÖŠ£¬ŹōÓŚ»ÆŗĻĪļµÄŹĒ
ABC
ABC
£ØĢīĶ¼ÖŠ×ÖÄø£©£®
¢ŪŌŚøĆ·“Ó¦ÖŠ£¬Éś³ÉCŗĶDµÄÖŹĮæ±ČĪŖ
44£ŗ7
44£ŗ7
£Ø¼ĘĖć½į¹ūÓĆ×ī¼ņÕūŹż±Č±ķŹ¾£©£®



 ±¦±“¼Ę»®ĘŚÄ©³å“Ģ¶į100·ÖĻµĮŠ“š°ø
±¦±“¼Ę»®ĘŚÄ©³å“Ģ¶į100·ÖĻµĮŠ“š°ø ÄÜæ¼ŹŌČ«ÄÜ100·ÖĻµĮŠ“š°ø
ÄÜæ¼ŹŌČ«ÄÜ100·ÖĻµĮŠ“š°ø

