

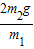
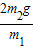 £» Ę«µĶ£»
£» Ę«µĶ£»

 ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 2m2g |
| m1 |
| 2m2g |
| m1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 £Ø2013?ŗ£µķĒųŅ»Ä££©µ±Ē°ŹŠŹŪÕäÖé·ŪµÄ¼Ūøń“ÓĪåŌŖµ½ÉĻ°ŁŌŖ²»µČ£¬µ«ĘäĶā¹Ū”¢ŹÖøŠ¶¼Ć»ÓŠĆ÷ĻŌ²īŅģ£®
£Ø2013?ŗ£µķĒųŅ»Ä££©µ±Ē°ŹŠŹŪÕäÖé·ŪµÄ¼Ūøń“ÓĪåŌŖµ½ÉĻ°ŁŌŖ²»µČ£¬µ«ĘäĶā¹Ū”¢ŹÖøŠ¶¼Ć»ÓŠĆ÷ĻŌ²īŅģ£®| ŹµŃéÄŚČŻ | ĻÖĻó | ½įĀŪ |
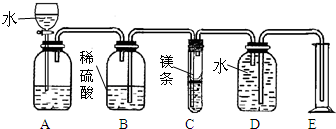
| £Ø1£©·Ö±š½«ÉŁĮæÓÅÖŹÕäÖé·ŪŗĶĮ®¼ŪÕäÖé·Ū·ÅČėŹŌ¹ÜÖŠ£¬¼ÓÉŁĮæĖ®£¬¾²ÖĆŅ»¶ĪŹ±¼äŗ󣬵Ī¼Ó ·ÓĢŖŹŌŅŗ ·ÓĢŖŹŌŅŗ £® |
Į®¼ŪÕäÖé·ŪµÄÉĻ²ćĒåŅŗ±äŗģ£¬ÓÅÖŹÕäÖé·ŪµÄČÜŅŗƻӊ±äÉ« | ²ĀĻė¢Ł³ÉĮ¢ |
| £Ø2£©·Ö±š½«ÉŁĮæÓÅÖŹÕäÖé·ŪŗĶĮ®¼ŪÕäÖé·Ū·ÅČėŹŌ¹ÜÖŠ£¬¼ÓÉŁĮæĖ®£¬¼ÓČČ£® | ÓÅÖŹÕäÖé·ŪµÄČÜŅŗÓŠ»ĘÉ«³öĻÖ£¬¾Ö²æ±äŗŚ£¬Į®¼ŪÕäÖé·ŪµÄČÜŅŗƻӊĆ÷ĻŌĻÖĻó | ²ĀĻė¢Ś³ÉĮ¢ |
| ÓÅÖŹÕäÖé·Ū | Į®¼ŪÕäÖé·Ū | |
| ÕäÖé·ŪµÄÖŹĮæ | 100 g | 100 g |
| ¼ÓČėŃĪĖįµÄÖŹĮæ | 460.0 g | 501.3 g |
| ÉÕ±ÖŠ×īÖÕĪļÖŹµÄ×ÜÖŹĮæ | 520.0 g | 557.7 g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā

| ÅäĮĻ±ķ | |
| °ü×Óʤ | Ćę·Ū”¢·¢“¼·Ū |
| ĻŚĮĻ | ĻŗČŹ”¢ŹŻČā”¢¼¦µ°”¢ °×²Ė”¢»ØÉśÓĶµČ |

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2012Äź¹óÖŻŹ”¹óŃōŹŠÖŠæ¼ŹŹÓ¦ŠŌæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com