
Ä³Ń§ÉśĪŖĮĖ²ā¶ØŹµŃéŹŅÖŠĀČĖį¼ŲѳʷµÄ“æ¶Č£¬Č”2.5gøĆѳʷÓė0.5g¶žŃõ»ÆĆĢ£¬¼ÓČČ»ģŗĻĪļT1Ź±¼äŗó£ØŌÓÖŹ²»²Ī¼Ó·“Ó¦£©£¬ĄäČ“”¢³ĘĮæŹ£Óą¹ĢĢåÖŹĮ棬ÖŲø“ŅŌÉĻ²Ł×÷£¬ŅĄ“ĪµĆ¼ÓČČT2”¢T3”¢T4Ź±¼äŗóŹ£Óą¹ĢĢåÖŹĮ棬¼ĒĀ¼Źż¾ŻČēĻĀ£ŗ
| ¼ÓČČŹ±¼ä | T1 | T2 | T3 | T4 |
| Ź£Óą¹ĢĢåÖŹĮæ£Øg£© | 2.12 | 2.08 | 2.04 | 2.04 |
£Ø1£©¼ÓČČT3Ź±¼äŗóĀČĖį¼ŲŹĒ·ńŅŃ¾ĶźČ«·“Ó¦ £»
£Ø2£©ĒóĶźČ«·“Ó¦ŗó²śÉśŃõĘųµÄÖŹĮ棻
£Ø3£©ĒóøĆѳʷ֊ĀČĖį¼ŲµÄÖŹĮæ·ÖŹż”£
£Ø1£©ŹĒ---------------------------------------------------£Ø1·Ö£©
£Ø2£©½ā£ŗÉčĶźČ«·“Ó¦ŗó²śÉśŃõĘųµÄÖŹĮæĪŖm
øł¾ŻÖŹĮæŹŲŗć¶ØĀÉmÖµĪŖ£ŗ 2.5g+0.5g==2.04g+m m==0.96g------------------£Ø1·Ö£©
(3) ½ā£ŗ Éčѳʷ֊ĀČĖį¼ŲµÄÖŹĮæX
|
|
245 ![]() 96
96
X 0.96 g
|
X=2.45g
ѳʷ֊ĀČĖį¼ŲµÄÖŹĮæ·ÖŹż=![]() ”Į100%=98%-----------------£Ø1·Ö£©
”Į100%=98%-----------------£Ø1·Ö£©



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
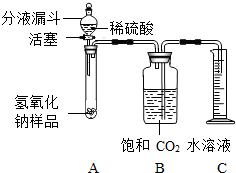
 Ä³Ń§ÉśĪŖĮĖ²ā¶ØŹµŃéŹŅÖŠŅ»ĘæŅņ±£“ę²»Éʶų²æ·Ö±äÖŹµÄĒāŃõ»ÆÄĘÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄ×°ÖĆ£ØĶ¼ÖŠĢś¼ÜĢØŅŃĀŌČ„£©£¬ŹµŃéŌŚ27”ę£¬101kPaĻĀ½ųŠŠ£¬ŹµŃé²½ÖčČēĻĀ£ŗ
Ä³Ń§ÉśĪŖĮĖ²ā¶ØŹµŃéŹŅÖŠŅ»ĘæŅņ±£“ę²»Éʶų²æ·Ö±äÖŹµÄĒāŃõ»ÆÄĘÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄ×°ÖĆ£ØĶ¼ÖŠĢś¼ÜĢØŅŃĀŌČ„£©£¬ŹµŃéŌŚ27”ę£¬101kPaĻĀ½ųŠŠ£¬ŹµŃé²½ÖčČēĻĀ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
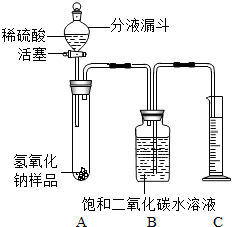
 Ä³Ń§ÉśĪŖĮĖ²ā¶ØŹµŃéŹŅÖŠŅ»ĘæŅņ±£“ę²»Éʶų²æ·Ö±äÖŹµÄĒāŃõ»ÆÄĘÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮĖČēĻĀĶ¼ĖłŹ¾µÄ×°ÖĆ£ØĶ¼ÖŠĢś¼ÜĢØŅŃĀŌČ„£©£¬ŹµŃéŌŚ27”ę£¬101kPaĻĀ½ųŠŠ£¬ŹµŃé²½ÖčČēĻĀ£ŗ
Ä³Ń§ÉśĪŖĮĖ²ā¶ØŹµŃéŹŅÖŠŅ»ĘæŅņ±£“ę²»Éʶų²æ·Ö±äÖŹµÄĒāŃõ»ÆÄĘÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮĖČēĻĀĶ¼ĖłŹ¾µÄ×°ÖĆ£ØĶ¼ÖŠĢś¼ÜĢØŅŃĀŌČ„£©£¬ŹµŃéŌŚ27”ę£¬101kPaĻĀ½ųŠŠ£¬ŹµŃé²½ÖčČēĻĀ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 Ä³Ń§ÉśĪŖĮĖ²ā¶ØŹµŃéŹŅÖŠŅ»ĘæŅņ±£“ę²»Éʶų²æ·Ö±äÖŹµÄĒāŃõ»ÆÄĘÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄ×°ÖĆ£ØĶ¼ÖŠĢś¼ÜĢØŅŃ¾ĀŌČ„£©£¬ŹµŃéŌŚ27”ę£¬101kPaĻĀ½ųŠŠ£¬ŹµŃé²½ÖčČēĻĀ£ŗ
Ä³Ń§ÉśĪŖĮĖ²ā¶ØŹµŃéŹŅÖŠŅ»ĘæŅņ±£“ę²»Éʶų²æ·Ö±äÖŹµÄĒāŃõ»ÆÄĘÖŠĢ¼ĖįÄʵÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮĖČēĶ¼ĖłŹ¾µÄ×°ÖĆ£ØĶ¼ÖŠĢś¼ÜĢØŅŃ¾ĀŌČ„£©£¬ŹµŃéŌŚ27”ę£¬101kPaĻĀ½ųŠŠ£¬ŹµŃé²½ÖčČēĻĀ£ŗ| ŹµŃé²½Öč | ŹµŃéĻÖĻó | ŹµŃé½įĀŪ |
| ²½ÖčŅ»£ŗ Ȕѳ£¬ČÜÓŚĖ®£¬µĪ¼Ó ¹żĮæµÄĀČ»ÆøĘČÜŅŗ Ȕѳ£¬ČÜÓŚĖ®£¬µĪ¼Ó ¹żĮæµÄĀČ»ÆøĘČÜŅŗ |
ÓŠ°×É«³Įµķ²śÉś ÓŠ°×É«³Įµķ²śÉś |
ĒāŃõ»ÆÄĘ²æ·Ö±äÖŹ |
| ²½Ö趞£ŗ ¾²ÖĆ£¬ĻņÉĻĒåŅŗÖŠµĪ¼Ó ·ÓĢŖ ¾²ÖĆ£¬ĻņÉĻĒåŅŗÖŠµĪ¼Ó ·ÓĢŖ |
±äŗģ ±äŗģ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ2006Äź½ĖÕŹ”Ģ©ÖŻŹŠĢ©ŠĖŹŠŃóĖ¼ÖŠŃ§¾ÅÄź¼¶»ÆѧŹµŃéÓėĢ½¾æ×ØĻīĮ·Ļ°ŹŌ¾ķ£Ø4ŌĀ·Ż£©£Ø½āĪö°ę£© ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com