
��������ƣ�Na2S2O3����һ����;�㷺�����ʡ�ij�����������Ʒ�к��������������ơ���ȡ16 g����Ʒ�����ձ��У�����113.6 gһ����������������ϡ����ǡ����ȫ��Ӧ���õ�120 g�����Ʋ�������Һ��
������Ӧ�Ļ�ѧ����ʽΪ��Na2S2O3 + H2SO4 === Na2SO4 + H2O + S��+ SO2��
����㣺
��1����Ʒ����������ƣ�Na2S2O3���������Ƶ������ȡ�
��2��������Һ����������������
���⡿�裺�����������Ϊx�������ɵĶ������������Ϊ2x��
16 g + 113.6 g = 120 g + x + 2x
x = 3.2 g
�裺�������������Ϊy�����ɵ�����������Ϊz��
Na2S2O3 + H2SO4 === Na2SO4 + H2O + S��+ SO2��
158 142 32 64
y z 3.2 g
y=15.8 g z=14.2 g
��1����Ʒ������������������Ƶ�������Ϊ��15.8 g : (16 g - 15.8 g) = 79:1
��2����������Һ��������������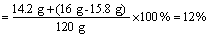
�𣺣�1����Ʒ������������������Ƶ�������Ϊ79:1��
��2����������Һ��������������Ϊ12%��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���������У�ȱ����ѧ���ݵ��ǣ�������
A�����������˿�Ԥ��ȱ����ƶѪ��������B���ú�̼�����Ƶ�ҩ������θ�����
�� C����ȼ�ŵ�ľ�������Ȼ���Ƿ�й¶����D���̬���ʲ��ܺͼ������ʻ��ʹ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����һƿ���õĹ���������Һ(��ǩ��ͼ��ʾ)����֪����������Һ�ڴ�Ź����лỺ���ֽ⡣ȡ����Һ34g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������33.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������3g��
����һƿ���õĹ���������Һ(��ǩ��ͼ��ʾ)����֪����������Һ�ڴ�Ź����лỺ���ֽ⡣ȡ����Һ34g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������33.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������3g��
����㣺
��1���ָ�ƿ��Һ����������������
��2���ø�ƿ��Һ����100g������������Ϊ3���Ĺ���������Һ��Ҫ����ˮ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijС��ͬѧ��2%��̼������Һ���뵽280 gʯ���飨ˮ���������ƵĻ����У�ʹ֮ǡ����ȫ��Ӧ�����ˣ�������Һ������Ϊ800g�����㷴Ӧ��������Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�����Һ�������У���ȷ���ǣ� ��
A����Һ������ɫ��
B����Һ��һ���ǻ����
C����Һ���Ǵ�����
D����Һһ���ǻ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�������ȫ���ǻ������ǣ� ��
A��������ʯ �͡���ˮ
�͡���ˮ
B��Һ��ʯ������ˮú��������
C��������ʯ��ʯ��ʯ��������
D���ƾ���Һ����ˮ����������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������գ�
��ѡ���ʣ�A. �����ʣ�B. С�մ�C. ���ۣ�D. ��ʯ�ң�E. ����أ�F. ����ͭ
��1������θ����� �� ��2��ʳƷ���еġ�˫������
��3��ţ���е���ҪӪ�������� ��4�������ڸ���������������
��5�����������Ʋ�����Һ������ ��6�������ĸ��Ϸ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com