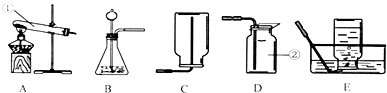
�������г��õ�������װ�ã��ش��й����⣺
��1��д����ͼ��ʾװ���б�����ŵ��������ƣ���
�Թ�
�Թ�
����
����ƿ
����ƿ
��
��2��ʵ��������A��Eװ����ȡ����ʱ������Ӧ�Ļ�ѧ����ʽΪ��
��ʵ��ʱ������ˮ���е�ˮ��Ϊ��ɫ����������Ϊ
�Թܿ�û�з�����
�Թܿ�û�з�����
��
��3����Bװ��������������װ�������Եķ�����
�ӳ���©����ע��ˮ����ˮ��û���¶˹ܿ�ʱ���õ��ɼУ���ס�����������ӵĽ�Ƥ�ܣ���֤�˴���©����Ȼ���ټ���������ˮ����ֹͣ��ˮ����©���е�ˮ�治���½�����˵����װ������������
�ӳ���©����ע��ˮ����ˮ��û���¶˹ܿ�ʱ���õ��ɼУ���ס�����������ӵĽ�Ƥ�ܣ���֤�˴���©����Ȼ���ټ���������ˮ����ֹͣ��ˮ����©���е�ˮ�治���½�����˵����װ������������
��С��ͬѧʵ������������������еĹ��壬�������
����
����
��ϴ�Ӹ������������ʵ���б����õ������������������е�
B
B
������ţ���
A�������� B����Ͳ C���ձ� D��©��
��4�����ȹ���̼�����ƻ����̼����臨��ܲ���CO
2���䷽��ʽ�ֱ���
NH
4HCO
3NH
3��+H
2O+CO
2�� 2NaHCO
3Na
2CO
3+H
2O+CO
2��
ijͬѧ���ü���̼�����Ƶķ�����ȡCO
2��Ӧ��ѡ�õķ���װ����
A
A
�����ɷ�ѡ�ü���̼�������ȡCO
2��������
�����ԣ���Ϊͬʱ�����������壬CO2�л����NH3
�����ԣ���Ϊͬʱ�����������壬CO2�л����NH3
��

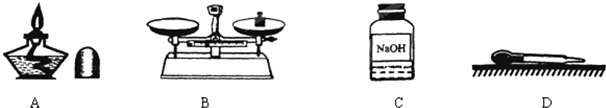
 �������г��õ�������װ�ã��ش��й����⣺
�������г��õ�������װ�ã��ش��й����⣺


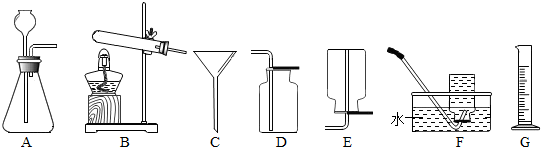
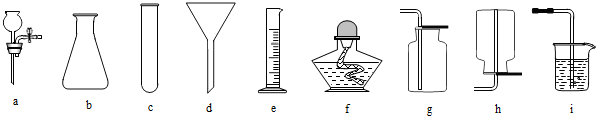

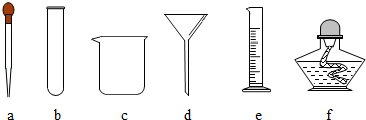 �������г��õ��������ش��й����⣺
�������г��õ��������ش��й����⣺