
 B”¢ĀĮÕō¹ų
B”¢ĀĮÕō¹ų C”¢øÖµ¼Ļß
C”¢øÖµ¼Ļß
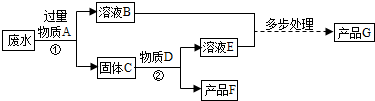
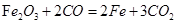
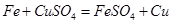 ”” A”¢B”¢C”¢E”¢G
”” A”¢B”¢C”¢E”¢G 2Fe+3CO2”£
2Fe+3CO2ӣ


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
| A£®C12+2NaI£½2NaCl+I2 | B£®I2+2KBr£½2KI+Br2 |
| C£®Br2+Na2S£½2NaBr+S”ż | D£®C12+K2S£½2KCl+S”ż |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¶¼ŹĒŅų°×É«µÄ¹ĢĢå | B£®ÓŠµ¼µēŠŌ |
| C£®ÄÜŗĶĻ”ĮņĖį·“Ó¦ | D£®Ķس£×“æöĻĀÄÜÓėŃõĘų·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

| A£®ĀĮĆĢŗĻ½šµÄĒæ¶ČŗĶÓ²¶Č±ČĀĮøß |
| B£®ĀĮĆĢŗĻ½š²»¾ßÓŠµ¼µēµ¼ČČŠŌ |
| C£®ĀĮµÄÖ±½Ó¹¹³ÉĪ¢Į£ŹĒĀĮ·Ö×Ó |
| D£®ŗĻ½šÖŠŌ×ÓÅÅĮŠ·½Ź½Óė“潚ŹōµÄŌ×ÓÅÅĮŠ·½Ź½ĻąĶ¬ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā


| A£®Ļņ·“Ó¦ŗóµÄ¹ĢĢåÖŠµĪ¼ÓĻ”ŃĪĖį£¬Ņ»¶ØÓŠĘųÅŻ²śÉś |
| B£®·“Ó¦ŗóµÄČÜŅŗÖŠŅ»¶ØƻӊĀČ»ÆŃĒĢś |
| C£®·“Ó¦ŗóµÄČÜŅŗÖŠŅ»¶ØƻӊĀČ»ÆĶ |
| D£®·“Ó¦ŗóµÄ¹ĢĢåÖŠŅ»¶Øŗ¬ÓŠCu”¢Fe”¢Zn |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
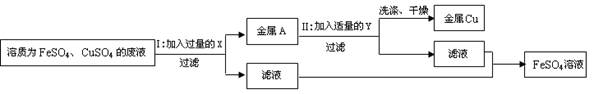
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā


²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com