
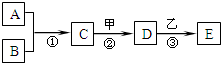
 32��A��E���ס��Ҿ��������л�ѧ�еij������ʣ�������һ�������µ�ת����ϵ����ͼ��ʾ����������������ȥ��������A��B���ǵ��ʣ������¾�Ϊ���壬B�ڿ����е��������ԼΪ21%����Ӧ�ٺ͢ھ��ų��������ȣ�D�ǿ������������ϵ�һ�ּ��ش�
32��A��E���ס��Ҿ��������л�ѧ�еij������ʣ�������һ�������µ�ת����ϵ����ͼ��ʾ����������������ȥ��������A��B���ǵ��ʣ������¾�Ϊ���壬B�ڿ����е��������ԼΪ21%����Ӧ�ٺ͢ھ��ų��������ȣ�D�ǿ������������ϵ�һ�ּ��ش�

 �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
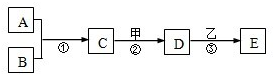 14��A��E���ס��Ҿ��������л�ѧ�еij������ʣ�������һ�������µ�ת����ϵ��ͼ��ʾ����������������ȥ��������A��B���ǵ��ʣ������¾�Ϊ���壬B�ڿ����е��������ԼΪ21%����Ӧ�ٺ͢ھ��ų��������ȣ�D�dz����ڸ������������ļ
14��A��E���ס��Ҿ��������л�ѧ�еij������ʣ�������һ�������µ�ת����ϵ��ͼ��ʾ����������������ȥ��������A��B���ǵ��ʣ������¾�Ϊ���壬B�ڿ����е��������ԼΪ21%����Ӧ�ٺ͢ھ��ų��������ȣ�D�dz����ڸ������������ļ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��E���ס��Ҿ��������л�ѧ�еij������ʣ�������һ�������µ�ת����ϵ��ͼ��ʾ����������������ȥ��������A��B���ǵ��ʣ������¾�Ϊ���壬B�ڿ����е��������ԼΪ21%����Ӧ�ٺ͢ھ��ų��������ȣ������������ЧӦ����Ҫ���壮
A��E���ס��Ҿ��������л�ѧ�еij������ʣ�������һ�������µ�ת����ϵ��ͼ��ʾ����������������ȥ��������A��B���ǵ��ʣ������¾�Ϊ���壬B�ڿ����е��������ԼΪ21%����Ӧ�ٺ͢ھ��ų��������ȣ������������ЧӦ����Ҫ���壮
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 A��E���ס��Ҿ��������л�ѧ�еij������ʣ�������һ�������µ�ת����ϵ��ͼ��ʾ����������������ȥ��������A��B���ǵ��ʣ������¾�Ϊ���壬B�ڿ����е��������ԼΪ21%����Ӧ�ٺ͢ھ��ų��������ȣ�D�ǿ������������ϵ�һ�ּ��ش�
A��E���ס��Ҿ��������л�ѧ�еij������ʣ�������һ�������µ�ת����ϵ��ͼ��ʾ����������������ȥ��������A��B���ǵ��ʣ������¾�Ϊ���壬B�ڿ����е��������ԼΪ21%����Ӧ�ٺ͢ھ��ų��������ȣ�D�ǿ������������ϵ�һ�ּ��ش��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com