
| �ⶨ���� | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
| ����Na2CO3��Һ/g | 10.0 | 20.0 | 30.0 | 40.0 | 50.0 |
| �������ʵ�������/g | 29.78 | 39.56 | 49.56 | 59.56 | δ�� |
| 73 |
| x |
| 44 |
| 0.44g |
| 106 |
| y��5.3% |
| 100 |
| 1g |
| 106 |
| 40g��5.3% |
| 117 |
| z |
| 2.34g |
| 59.56g-1g |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
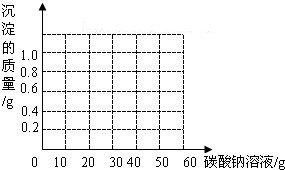
 ij��ͬѧ�����ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ�˶Է�Һ���д�����ijͬѧ��������ʵ�飺ȡ��Һ���ϲ���Һ20.0g���ձ��У���ε���������������Ϊ5.3%��̼������Һ������������̼������Һ������/g�������ɳ�����������/g���ı仯��ϵ��ͼ��ʾ������������ȷ��0.1%��
ij��ͬѧ�����ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ�˶Է�Һ���д�����ijͬѧ��������ʵ�飺ȡ��Һ���ϲ���Һ20.0g���ձ��У���ε���������������Ϊ5.3%��̼������Һ������������̼������Һ������/g�������ɳ�����������/g���ı仯��ϵ��ͼ��ʾ������������ȷ��0.1%���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
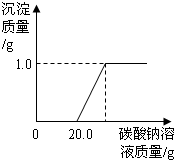
 ��2006?������һģ��ij��ͬѧ�����ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ�˶Է�Һ���д�����ijͬѧ��������ʵ�飺ȡ��Һ�����ϲ���Һ40mL���ձ��У���ε���Na2CO3��Һ����������¼����Na2CO3��Һ������x�����й����ı仯��ϵ��ͼ��ʾ��ͼ�������꣨y����ʾ
��2006?������һģ��ij��ͬѧ�����ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ�˶Է�Һ���д�����ijͬѧ��������ʵ�飺ȡ��Һ�����ϲ���Һ40mL���ձ��У���ε���Na2CO3��Һ����������¼����Na2CO3��Һ������x�����й����ı仯��ϵ��ͼ��ʾ��ͼ�������꣨y����ʾ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
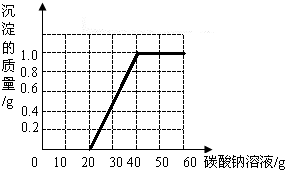
 ij��ͬѧ�����ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ�˶Է�Һ���д�����ijͬѧ��������ʵ�飺ȡ��Һ���ϲ���Һ20.0g���ձ��У���ε���������������Ϊ5.3%��̼������Һֱ������������̼������Һ�����������ɳ����������ı仯��ϵ��ͼ��ʾ��
ij��ͬѧ�����ꡰ������̼����ȡ�����ʡ�ʵ���Һ����ʢ�д������������Ȼ��ƵĻ����Һ���������������ʣ���Ϊ�˶Է�Һ���д�����ijͬѧ��������ʵ�飺ȡ��Һ���ϲ���Һ20.0g���ձ��У���ε���������������Ϊ5.3%��̼������Һֱ������������̼������Һ�����������ɳ����������ı仯��ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɽ��ʡ�Ͳ������п���ѧģ���Ծ��������������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com