
�ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᡣ������
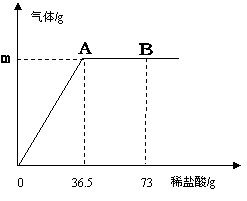
�μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g�����������������ȫ���ݳ�����������������������ϡ�����������ϵ����ͼ��ʾ���Լ��㣺
��1��A��������������m=_________��
��2��������̼���Ƶ����������������ȷ��0.1%����
��3��B��ʱ���ձ�����Һ�е�������__________��д����ѧʽ����
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g�����������������ȫ���ݳ�����������������������ϡ�����������ϵ����ͼ��ʾ���Լ��㣺
�ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g�����������������ȫ���ݳ�����������������������ϡ�����������ϵ����ͼ��ʾ���Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
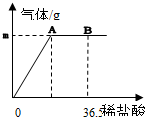 �ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g�����������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺
�ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g�����������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣬ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g���������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺
�ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣬ������μ���36.5gʱ���ձ�����Һ��������Ϊ40.3g���������������ȫ���ݳ�����������������������ϡ�����������ϵ��ͼ��ʾ���Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?������ģ�⣩�ҹ�����ij�κ������Ĵ����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������������������ϡ�����������ϵ����ͼ��ʾ���������������ȫ���ݳ�����������μ���ͼA��ʱ���ձ�����Һ��������Ϊ40.3g�����Լ��㣺
��2011?������ģ�⣩�ҹ�����ij�κ������Ĵ����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������ձ��в�����ϡ���ᣮ������������������ϡ�����������ϵ����ͼ��ʾ���������������ȫ���ݳ�����������μ���ͼA��ʱ���ձ�����Һ��������Ϊ40.3g�����Լ��㣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?��������ģ���ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������Һ����������μ���CaCl2��Һ�������������ʵ���������CaCl2����������ϵ��ͼ��ʾ��
��2013?��������ģ���ҹ�����ij�κ������Ĵ��Na2CO3����Ʒ�г������������Ȼ��ƣ��������ʺ��Բ��ƣ���Ϊ�ⶨ�ò�Ʒ��̼���Ƶ������������ֳ�ȡ6g���������Һ����������μ���CaCl2��Һ�������������ʵ���������CaCl2����������ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com