
���ͼ�ش�����

(1)�������ƣ���_____����_____��
(2)������غͶ�����������������ѡ��װ��_____(ѡ�����)�� E ��ϣ���ѧ����ʽ Ϊ_____���������̵�������_____��
(3)ʵ���ҿ���װ��B��C�� H2��C װ�������B���ŵ���_____����Dװ���ռ� H2������Ӧ��_____(ѡ�a����b��)��ͨ�롣
(4)F Ϊ����ˮװ�ã��൱�ڻ�ѧʵ���е�_____װ�á����װ�ô�������ˮ�У�������������ˮ�������϶ม����˵����ˮ_____(�Ӳ��������)����Ҫ�õ���ˮ������ Ҫ����_____(���������)��
�ƾ��� ��Һ©�� A 2KClO32KCl+3O2�� ������ ���Կ��Ʒ�Ӧ�ķ�����ֹͣ a ���� Ӳ ���� ����������1����������̨�����dz���©������2�����ȹ�����ȡ����ѡ����װ��A��������غͶ���������ȡ�����Ļ�ѧ����ʽΪ��2KClO3 2KCl+3O2�������ж�������������á���3��Cװ�������B���ŵ��ǿ���ʱ���Ʒ�Ӧ�ķ�����ֹͣ����Eװ���ռ�H2�������������ܶȱȿ������ܶ�С����...
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ������2018����꼶�п���Ӧ��ѵ����ѧ�Ծ� ���ͣ���ѡ��
��H2SO4��CuSO4��100.0g�����Һ�м���12.0g���ۣ���ַ�Ӧ����й�Һ���룬�õ�a g��Һ��12.0g���ܹ��壬��ù����м�������ϡ���ᣬ����0.2g H2������˵����ȷ����
A. a = 100.0
B. �ù�����ȫ���ܽ���ϡ������
C. ag��Һ�к�����Ԫ�ص�����Ϊ6.4g
D. ԭ�����Һ��H2SO4��CuSO4��������Ϊ7��40
C ��������A.�������غ㶨�ɿ�֪����Ӧǰ�����ʵ����������䣬 ��Ӧ����Һ����������a = 100.0g+12.0g- 12.0g-0.2g<100g������ B. �費��������������Ϊx Fe +2HCl== FeCl2+H2�� 56 2 x 0.2g 56/x=2/0.2g x=5.6g ��Ӧ��������ͭ������6.4g��ͭ�������Ӧ���ù��岻��ȫ���ܽ���ϡ...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������С�������ѧ���ϴ���ѧ��ʵ����ѧ��У����2018����꼶��ѧ�ڵ������¿���ѧ�Ծ� ���ͣ������
�ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧ���е�˼ά��ʽ�����ݵ��ˮ��ʵ�飬�ش��������⡣
��1���Ӻ���Ϲ۲죺��ͼ��ʾ��b�в�����������_________���ѧʽ����
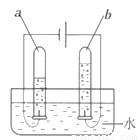
��2�������Ϸ���������˵����ȷ����_____������ĸ����
A��ˮ����������������ɵ�
B��ˮ������ԭ�Ӻ���ԭ�ӹ��ɵ�
C��ÿ��ˮ��������������ԭ�Ӻ�һ����ԭ�ӹ��ɵ�
��3���ӷ����ϱ�ʾ�����ˮ�Ļ�ѧ����ʽΪ______________________________��
O2 C 2H2O2H2��+O2�� ����������1�����ˮʱ�����Դ����������һ�˲���������������2��A��ˮ������Ԫ�غ���Ԫ����ɵģ�����B��ˮ����������ԭ�Ӻ���ԭ�ӹ��ɵģ�����C��ÿ��ˮ��������������ԭ�Ӻ�һ����ԭ�ӹ��ɵģ���ȷ����ѡC����3�����ˮʱ�����������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������С�������ѧ���ϴ���ѧ��ʵ����ѧ��У����2018����꼶��ѧ�ڵ������¿���ѧ�Ծ� ���ͣ���ѡ��
���ɷ����ڿ�����һ��ʱ�䣬��ò����ɴ࣬˵�������к��� �� ��
A. ˮ���� B. ������̼ C. ���� D. ����
A �����������ɷ����ڿ�����һ��ʱ�䣬��ò����ɴ࣬�DZ������տ����е�ˮ���������⣬��ѡA���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר��13 ʵ�鷽���ĸĽ���Ԥ���⣩-��ʤ2018�п���ѧѹ����ȫ���ؾ�Ʒ ���ͣ���ѧ̽����
ij��ȤС��ͬѧȥ�γ��ιۣ������˲��ִ�����Ʒ�����������������̽����
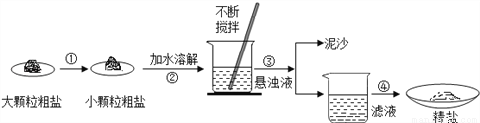
(1)�����۵�������_________________
(2)���������õ�������������Ϊ_____________________��
(3)ʵ������������õľ��Σ������㾫�ε��Ƶ��ʣ������Ƶ��ʽϵͣ������ԭ����__________(�����)��
A��ʳ��û��ȫ���ܽ⼴���� B������ʱʳ�ηɽ�����
C�����������þ��κܳ�ʪ D��������մ�еľ���ûȫ��ת�Ƶ�����ֽ�ϡ�
(4)�������ϵ�֪�������г���������ɳ�Ȳ����������⣬�����������Ŀ���������(�ٶ�����������ֻ��MgCl2һ��)��Ϊ�˵õ��ϴ������Ȼ��ƣ�С��ͬѧ�����õġ����Ρ����������´�����
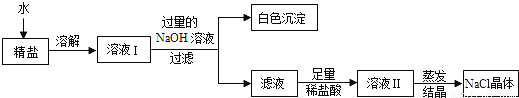
�ٰ�ɫ�����Ļ�ѧʽΪ_______��
���ڵõ�����Һ�м���������ϡ�����Ŀ����____________________��
���� ���裬��ֹ�ֲ��¶ȹ������Һ��ɽ� ABD Mg(OH)2 ��ȥ�������������� ����������1�����˿��Խ�������Ϳ�������룬���Բ����۵������ǹ��ˣ���������������Ҫ�õ�������������Ϊ���裬��ֹ�ֲ��¶ȹ������Һ��ɽ�����3��A��ʳ��û��ȫ���ܽ⼴���ˣ��ᵼ��ֻ����ƫ�ͣ���ȷ��B������ʱʳ�ηɽ����ң��ᵼ���Ƶ���ƫ�ͣ���ȷ����C�����������þ��κܳ�ʪ���ᵼ���Ƶ���ƫ�ߣ�����D��������...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר��13 ʵ�鷽���ĸĽ���Ԥ���⣩-��ʤ2018�п���ѧѹ����ȫ���ؾ�Ʒ ���ͣ�ʵ����
С����ľ̿�ۻ�ԭ����ͭ����ȡͭ����Ӧ��ѧ����ʽΪ��______��
������̼�Ļ�ѧ���ʷ�������Ϊ��ʵ���п��ܻ���һ����̼������
���������ϡ�һ����̼������ʹʪ��Ļ�ɫ�Ȼ�����ֽ����ɫ��
�����ʵ�顿С���������ͼ��ʾ��ʵ��װ�ã�������ʵ����֤��

(1)Aװ���оƾ��Ƽ����ֵ�Ŀ����_______��
(2)���þƾ��Ƹ��Թ�A����ʱ��Bװ���о����̲������ݣ���Һ�岢û�л��ǵ�������֣�ԭ����______��
(3)C��ʪ���ɫ�Ȼ�����ֽ������˵����_____�������ɣ�֤��С�����ж�����ȷ�ģ������ʵ������������ԭ�������(дһ��)��______��
(4)���װ��ʱ��Ҫ���ǻ������أ���ˣ�Ҫ��װ��C�Ҳർ�ܿ�����____װ�ã�
(5)����Ӧ������С������һ�����⣺����ֹͣ���ȣ�______�������Թ�ը�ѣ�������ȥ��Bװ�ã���ᵼ��________��������������һ��ʵ����������Թ��л�ԭ����ͭ������_________��
C+2CuO2Cu+CO2�� ʹ���漯�У�����¶� �ռ���ʱ���Թ��ڵĿ����������ʹӵ��ܿ��ݳ� CO ľ̿���Թ��ڿ����е�������Ӧ����CO(��ľ̿�뷴Ӧ�����ɵ�CO2��������CO β������ Bװ���ڵ�ʯ��ˮ�ᵹ�����Թ��� �Թ��к��ȵ�ͭ�������е�������������������ͭ ���õ��ɼмн�A��Bװ�ü����Ƥ�ܣ���Ϩ��ƾ��Ʋ���ȥB��Cװ�ã����Թ���ȴ���ٽ��Թ��еķ�ĩ���� ���������ڸ���������̼�ܻ�...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�����2018����꼶��ѧ�����п������п��Ի�ѧ�Ծ� ���ͣ�������
Ϊ�ⶨij��ˮ���������Ƶ�����������С��ȡ40 g��ˮ��Ʒ���뵽��ƿ�У���μ���10%��ϡ���ᣬ��ǡ����ȫ��Ӧʱ������ϡ����49 g�����ˮ���������Ƶ�����������
10% ����������40g��Ʒ���������Ƶ�����Ϊx H2SO4��2NaOH��Na2SO4��2H2O 98 80 49 x =���x=4g ����������Һ�����ʵ���������Ϊ=10% ����Ʒ���������Ƶ���������Ϊ10%���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�����2018����꼶��ѧ�����п������п��Ի�ѧ�Ծ� ���ͣ���ѡ��
ijЩ��ѧС���������֤Fe��Cu��Ag�Ľ������˳������ַ�������������ѡ�õ��Լ����£����в����е��ǣ� ��
A��Fe��CuSO4��Һ��Ag
B��FeCl2��Һ��Cu��AgNO3��Һ
C��Ag��CuSO4��Һ��FeSO4��Һ
D��Fe��Cu��AgNO3��Һ������
C ���������������� A�������û�������ͭ�е�ͭ���ó��������������ͭ���������û�������ͭ�е�ͭ���ó��������ͭ������������֤Fe��Cu��Ag�Ľ������˳��A��ȷ�� B��ͭ�����û����Ȼ������е��������û����������е�����˵�����������������ͭ��ͭ������������֤Fe��Cu��Ag�Ľ������˳��B��ȷ�� C���������û�������ͭ�е�ͭ�����������е������ܵó�����ͭ�Ļ�...�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ����ɽ��2018����꼶�ڶ�ѧ�ڶ�ģ��ѧ�Ծ� ���ͣ���ѡ��
��ѧ������������Ӧ�ù㷺�����й������ڻ�ѧ�仯���ǣ� ��
A. ��ˮɹ�� B. ����̿��ˮ C. ú��ú�� D. �ɱ�����
C ��������A����ˮɹ�ι�����û�����������ɣ����������仯��B������̿��ˮ������û�����������ɣ����������仯��C��ú��ú�������������������ɣ����ڻ�ѧ�仯�� D���ɱ����������ֻ��״̬�����ı䣬û�����������ɣ����������仯����ѡC���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com