
��4�֣���֪̼�����Ƽ����ֽܷ�Ϊ̼���ơ�ˮ�Ͷ�����̼����̼�������Ȳ��ֽ⡣����ij������������һ��̼�������л���������̼���ƣ�ȡ4.0g ��Ʒ���ȵ��������ټ���ʱ�����ռ���������̼����0.88g��
��1��д��̼���������ȷֽ�Ļ�ѧ����ʽ ��
��2��Ҫ�õ�����Ķ�����̼���壬��ͼ��ʢ������ˮ��Ũ���ᣬ
���ɵ�����Ӧ�� ��ѡ�a����b�����˵��롣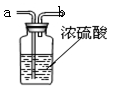
��3�������Ʒ��̼���Ƶ�������������д��������̣�
��1��2NaHCO3 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��2��a ��3��16%
�������������
��1�����������֪��̼�����Ƽ����ֽܷ�Ϊ̼���ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��2NaHCO3��Na2CO3��H2O��CO2����
��2��Ҫ�ﵽ�����Ч�������ɵ�����Ӧ�ӳ��ܽ��룬�Ӷ̹��е���������Ӧ��a�˵��롣
��3�����������֪����֪��Ϊ������̼��������δ֪��Ϊ����Ʒ��̼���Ƶ�����������
����˼·���ɸ��ݶ�����̼��������������̼�����Ʒֽ�Ļ�ѧ����ʽ�������Ӧ��̼�����Ƶ���������һ�����δ֪�����������������£���д��������̣�
�⣺��̼�����Ƶ�����Ϊx��
2NaHCO3��Na2CO3��H2O��CO2��
168 44
x 0.88g
168��44=x��0.88g
x= 3.36g
����Ʒ��̼���Ƶ���������Ϊ��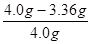 ��100%=16%
��100%=16%
�𣺸���Ʒ��̼���Ƶ���������Ϊ16%
���㣺��ѧ����ʽ����д������ľ������ۺϼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
���ˮʵ���У�Ϊ������ˮ�ĵ����ԣ�����ˮ�м���һ������ϡ���ᣮ
��1��ʵ������У����Դ���������IJ������ڲ������������� ����
��2��ij��ʵ���У���10��10.8%��ϡ���ᵹ��ʢ��98��ˮ���ձ��л�Ͼ��ȵ�A��Һ����A��Һ�����ʵ���������Ϊ ��
��3����A��Һȫ��������ˮװ�ã���ͨ�練Ӧ����ʣ��Һ�еĺ�ˮ��Ϊ98%ʱ�����Ƶö��ٿ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ijͬѧΪ�˲ⶨNaCl��MgCl2����������MgCl2��������������������ʵ�飺��80g������������ˮ�����Һ��ƽ���ֳ��ķݣ��ֱ������ͬ����������NaOH��Һ���������ʵ�����ݣ�
| ʵ����� | һ | �� | �� | �� |
| ���������������g�� | 20 | 20 | 20 | 20 |
| ����NaOH��Һ��������g�� | 20 | 40 | 60 | 80 |
| ���ɳ�����������g�� | 2��9 | m | 8��7 | 8��7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��8�֣�ͬѧ�Ƕ�ʵ�顰����ʯ��ʯ��չ������̽����
���ϣ�ʯ��ʯ�е����ʸ��²��ֽ��Ҳ���ϡ���ᷴӦ
��1��Ϊ֤��ʯ��ʯ�ѷֽ⣬��λͬѧ��Ʒ������£�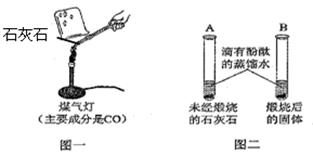
��ͬѧ��ͼһ��ʾ����ʵ�飨ú�������ܴﵽʯ��ʯ�ֽ���¶ȣ����۲쵽�ձ��ڱ�ʯ��ˮ����ǣ�д��ʯ��ˮ������Ӧ�Ļ�ѧ����ʽ ��
II����ͬѧ��ͼ����ʾ�������飬�۲쵽B��Һ��� ɫ��Aʵ������� ��
III����ͬѧȡһ������Ϊ12g��ʯ��ʯ���գ�һ��ʱ����ֹ�����������2��2g��֤��ʯ��ʯ�ѷֽ⡣
IV�������Ϊ��ͬѧ�ķ����������������ԭ�� ��
��2��Ϊ�ⶨʯ��ʯ�Ĵ��ȣ���ͬѧ�����ղ����պ�Ĺ����������ϡ�����У��ֲ���2.2g���壬����������ݼ��㣺����ȡ�����ʯ��ʯ��̼��Ƶ����������Ƕ��٣�
�������д�ڴ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ʵ������һƿ������ķ�Һ��������·����ⶨ�÷�Һ�����������������ȡ����Ϊ18.2g�ĽྻС�ձ��������е���һ���������Һ�������������Ϊ33.2g��Ȼ��һö����Ϊ10.8g������������ɰֽ��ĥȥ�����⣬�����������Һ�е��������ʷ�Ӧ�������С�ձ��г�ַ�Ӧ�����������治�������ݲ���������������Ϊ43.9g���Լ���ԭ�����Һ�����������������������������С�����һλ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
����̼���ơ�(2Na2CO3��3H2O2)�׳ƹ���˫��ˮ��������̼���ε����ʣ�����˫��ˮ�IJ��ȶ��Ժ������ԣ��Ǻܺõ����������������㷺Ӧ����ϴ�ӡ�ӡȾ��ҽҩ��������̼���ơ����Ʊ��������£�
��ش��������⣺
��1��H2O2�ڴ��������������ֽ⣬˵����ѧ�仯���ٶȺ� ��أ�
��2���ڡ���̼���ơ��м�����������ʱ�����ᵼ�¡���̼���ơ����ʵ��� ������ţ���
| A���������� | B��ϡ���� | C�������� | D��ʯ��ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�ҹ��ɹ�����ʳ��������м�ǧ����ʷ����������[�������ǣ�C6H12O6��Ϊ��]�ڷ���ʱ��Ӧ�����Ҵ���C2H6O���Ͷ�����̼���仯ѧ��Ӧ����ʽΪ��C6H12O6 = 2C2H6O + 2CO2���������ͺ�����90kg����ʳ�����������ɵİƺ��Ҵ�����ǧ�ˣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ֽ����������������Ƶķ�ˮ���辭���������Ժ��ŷš�Ϊ�ⶨ�˷�ˮ���������Ƶ�����������������Աȡ��40g��ˮ��Ʒ���뵽��ƿ�У���μ���10%��ϡ���ᣬ��ǡ����ȫ��Ӧʱ������ϡ����49 g�������ˮ���������Ƶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ij������Ҫ280g��ʯ�ң�CaO�������������Ҫ̼��Ƶ������Ƕ��٣�������Ҫ��̼���80%��ʯ��ʯ�����ʲ��μӷ�Ӧ���������Ƕ��٣���CaCO3 CaO+CO2����
CaO+CO2����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com