

���� ��1������100g������������Ϊ12%��NaCl��Һ�IJ�������ֱ��ǣ����㡢�������ܽ⣬���ݸ���������Ҫʹ�õ��������ж�����Ҫ�IJ���������
��2��������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ�ݴ˽��з������
��3������ͼ�ڡ�ͼ�۲��������з������
��4��������������=��Һ���������ʵ��������������з������
��5������Ȼ����к����������ܵ����ʣ������ʵ����ȡ�����ʵ�����ƫС������ܼ�����=��Һ����-�������������з������
��� �⣺��1������100g������������Ϊ12%��NaCl��Һ�����ȼ���������Һ����NaCl��ˮ���������ٳ��������NaCl����ȡˮ���������ܽ⣻����Щ��������Ҫ�����������ƿ��������ƽ��ҩ�ס���Ͳ���ձ��Ͳ��������������ڲ����������ǹ��ƿ�����������ձ�����Ͳ��
��2��������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ��ͼ�����Ȼ��ƺ������λ�÷ŷ��ˣ�
��3��ͼ�ڡ�ͼ�۲����ֱ��dz������ܽ⣮
��4����������=��Һ���������ʵ���������������100g������������Ϊ12%��NaCl��Һ�������Ȼ��Ƶ�����Ϊ100g��12%=12g��
��5������Ȼ����к����������ܵ����ʣ������ʵ����ȡ�����ʵ�����ƫС�������ʵ�����������ƫС��
����ˮ������Ϊ100g-12g=88g����88mL����ѡȡ��Ͳʱ������ѡ����һ����ȡ����С������Ͳ��Ӧ��100mL��Ͳ��ȡ88mLˮ��
�ʴ�Ϊ����1�����������ձ�����2��ͼ�����Ȼ��ƺ������λ�÷ŷ��ˣ���3���������ܽ⣻��4��12����5��ƫС���ۣ�
���� �����ѶȲ�����ȷ����һ����������������Һ�ķ�����ʵ�鲽�衢�������������ȷ�����Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | �� �� | ѡ���Լ� | �� �� |
| A | CO2��HCl�� | ����NaHCO3��Һ | ϴ�� |
| B | Cu��CuO�� | H2O | ����������ˮ������ܽ⣬���ˣ�ϴ�� |
| C | NaCl���壨Na2CO3�� | ϡ���� | �������������ᣬ���� |
| D | CaCl2��Һ��KCl�� | ��Na2CO3��Һ ��ϡ���� | �ȼ�������Լ��٣�����ϴ�ӣ����������м��������Լ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
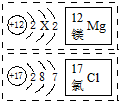
| A�� | þԭ�ӽṹͼ��X=8 | B�� | ��Ԫ�ص�ԭ������Ϊ17 | ||
| C�� | þ�ǽ���Ԫ�ء����Ƿǽ���Ԫ�� | D�� | þԭ����ʧȥ���Ӵ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ֻ��MgCl2��û��NaCl | B�� | һ��û��CuCl2��Na2CO3 | ||
| C�� | ������MgCl2��KNO3 | D�� | һ������CaCl2��NaCl��MgCl2��ɵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com