
 ��ͼΪijʵ����ʢ��ϡ�����Լ�ƿ��Ϊ�ⶨ������������ȡ6g��Һ���Թ��У���������εμ�10%������������Һ����pH��ֽ���ϲ�����Һ�����ȣ����μ���4gʱ����ҺpHֵǡ��Ϊ7��
��ͼΪijʵ����ʢ��ϡ�����Լ�ƿ��Ϊ�ⶨ������������ȡ6g��Һ���Թ��У���������εμ�10%������������Һ����pH��ֽ���ϲ�����Һ�����ȣ����μ���4gʱ����ҺpHֵǡ��Ϊ7��| 36.5 |
| x |
| 40 |
| 0.4g |
| 40 |
| 0.4g |
| 58.5 |
| y |
| 0.365g |
| 6g |
| 0.585g |
| 6g+4g |


 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ���������������꼶��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�������
��ͼΪijʵ����ʢ��ϡ�����Լ�ƿ��Ϊ�ⶨ������������ȡ6g��Һ���Թ��У���������εμ�10%������������Һ����pH��ֽ���ϲ�����Һ�����ȣ����μ���4gʱ����ҺpHֵǡ��Ϊ7��
��1����pH��ֽ�ⶨ��Һ�����ȵľ��巽������ ����
��2���Լ��㣺
��ϡ���������������
���Թ���������Һ���������������ⶨ��Һ��������ɵ���Һ������ʧ���Բ��ƣ���������ȷ��1%��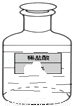
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼΪijʵ����ʢ��ϡ�����Լ�ƿ��Ϊ�ⶨ������������ȡ6g��Һ���Թ��У���������εμ�10%������������Һ����pH��ֽ���ϲ�����Һ�����ȣ����μ���4gʱ����ҺpHֵǡ��Ϊ7��
��ͼΪijʵ����ʢ��ϡ�����Լ�ƿ��Ϊ�ⶨ������������ȡ6g��Һ���Թ��У���������εμ�10%������������Һ����pH��ֽ���ϲ�����Һ�����ȣ����μ���4gʱ����ҺpHֵǡ��Ϊ7���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ���������������꼶���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com