
 �T
�T
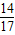 ×100%�T0.13g��
×100%�T0.13g��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ������ֲ��á��Ƕ�����ֽ����еĵ����ʣ���ԭ���ǰѵ������еĵ�Ԫ����ȫת���ɰ�������ѧʽΪ��NH3��������ϡ�������հ�������Ӧ�Ļ�ѧ����ʽ��
Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ������ֲ��á��Ƕ�����ֽ����еĵ����ʣ���ԭ���ǰѵ������еĵ�Ԫ����ȫת���ɰ�������ѧʽΪ��NH3��������ϡ�������հ�������Ӧ�Ļ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �ֽ� |
| ||
| ˮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����
�����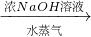 ����Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ�����ȡ30mLţ���øǶ�����뵰���ʣ��ѵ�Ԫ����ȫת���ɰ���NH3������50g4.9%ϡH2SO4��Һ���պ�ʣ�������Ҫ��38.0g4.0%��NaOH��Һ�кͣ���
����Ϊ�˲ⶨijţ����Ʒ�е����ʵĺ�����ȡ30mLţ���øǶ�����뵰���ʣ��ѵ�Ԫ����ȫת���ɰ���NH3������50g4.9%ϡH2SO4��Һ���պ�ʣ�������Ҫ��38.0g4.0%��NaOH��Һ�кͣ����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com