��ѧ����ǧ������д�����з�Ӧ�Ļ�ѧ����ʽ������д����������Ӧ���ͣ�
��1����˿��������ȼ��________ ����________��Ӧ��
��2��˫��ˮ������________��
��3����¯�����У�һ����̼���������ڸ����·�Ӧ�������Ͷ�����̼________��
��4���ձ��˵�վ��ը��Ҫ��Դ��ˮ�ڸ����������ﯣ�Zr��������Ӧ��������ﯣ�ZrO2���������������������϶�������ը����д��ˮ��ﯷ�Ӧ�Ļ�ѧ����ʽ________�������������ϱ�ը�Ļ�ѧ����ʽ________��
�⣺��1����˿��������ȼ��������������������Ӧ�Ļ�ѧ����ʽΪ��3Fe+2O
2
Fe
3O
4���÷�Ӧ���ϡ����һ�������������ڻ��Ϸ�Ӧ��
��2�����������ڶ������̵Ĵ�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H
2O
2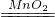
2H
2O+O
2����
��3����ҵ����һ����̼��ԭ��������������Ҫ������CO�Ļ�ԭ�ԣ��ڸ����º���������Ӧ�������Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪFe
2O
3+3CO

2Fe+3CO
2��
��4��ˮ�ڸ����������ﯣ�Zr��������Ӧ��������ﯣ�ZrO
2������������Ӧ�Ļ�ѧ����ʽΪZr+2H
2O

ZrO
2+2H
2�������������϶�������ը����Ӧ�Ļ�ѧ����ʽΪ2H
2+O
2
2H
2O��
�ʴ�Ϊ����1��3Fe+2O
2
Fe
3O
4�����ϣ���2��2H
2O
2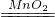
2H
2O+O
2������3��Fe
2O
3+3CO

2Fe+3CO
2����4��Zr+2H
2O

ZrO
2+2H
2��2H
2+O
2
2H
2O��
���������ȸ��ݷ�Ӧԭ���ҳ���Ӧ��������Ӧ���������ݻ�ѧ����ʽ����д���������������д���ٸ��ݷ�Ӧ����ȷ����Ӧ���ͣ�
�����������ѶȲ�����ѧ�����ݷ�Ӧԭ����д��ѧ����ʽ���ж���Ӧ���͵����������ջ�ѧ����ʽ����д���������ֻ�����Ӧ���͵�����������ȷ����⣮

 Fe3O4���÷�Ӧ���ϡ����һ�������������ڻ��Ϸ�Ӧ��
Fe3O4���÷�Ӧ���ϡ����һ�������������ڻ��Ϸ�Ӧ��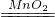 2H2O+O2����
2H2O+O2���� 2Fe+3CO2��
2Fe+3CO2�� ZrO2+2H2�������������϶�������ը����Ӧ�Ļ�ѧ����ʽΪ2H2+O2
ZrO2+2H2�������������϶�������ը����Ӧ�Ļ�ѧ����ʽΪ2H2+O2 2H2O��
2H2O�� Fe3O4�����ϣ���2��2H2O2
Fe3O4�����ϣ���2��2H2O2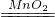 2H2O+O2������3��Fe2O3+3CO
2H2O+O2������3��Fe2O3+3CO 2Fe+3CO2����4��Zr+2H2O
2Fe+3CO2����4��Zr+2H2O ZrO2+2H2��2H2+O2
ZrO2+2H2��2H2+O2 2H2O��
2H2O��


 ��1���������̺���ѧ�����Ĵ���ѧ�ʣ��������������գ�
��1���������̺���ѧ�����Ĵ���ѧ�ʣ��������������գ�