


 ������Ӧ���ϵ�д�
������Ӧ���ϵ�д� ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
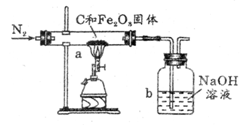
 ij��ѧ��ȤС���ͬѧΪ�ⶨ�ٻƽ�ͭп�Ͻ����Ԫ�ص�������������ȡ20�˼ٻƽ������ձ��У���ijŨ�ȵ�ϡ����50�ˣ���5�μ��룬ÿ�γ�ַ�Ӧ��ȡ�����壬�����ˡ�����Ȳ����������5��ϡ����������ʣ�����������¼���£�
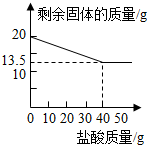
ij��ѧ��ȤС���ͬѧΪ�ⶨ�ٻƽ�ͭп�Ͻ����Ԫ�ص�������������ȡ20�˼ٻƽ������ձ��У���ijŨ�ȵ�ϡ����50�ˣ���5�μ��룬ÿ�γ�ַ�Ӧ��ȡ�����壬�����ˡ�����Ȳ����������5��ϡ����������ʣ�����������¼���£�| ʵ����� | ϡ�����������g�� | ʣ������������g�� |
| 1 | 10 | 18.375 |
| 2 | 10 | 16.750 |
| 3 | 10 | 15.125 |
| 4 | 10 | 13.500 |
| 5 | 10 | 13.500 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
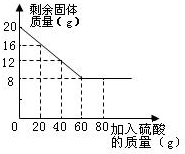 ij��ѧ��ȤС���ͬѧΪ�ⶨCu��CuO�����Ʒ��CuO�ĺ������ס��ҡ���������λͬѧ���ȳ�ȡ20g��Ʒ���ձ��У��ڷֱ����20g��40g��60g��80g��������������ͬ��ϡ���ᣮ��ַ�Ӧ���й���ʣ�࣮�����ˡ�����������ʣ�������������ͼ��ʾ���ش��������⣺
ij��ѧ��ȤС���ͬѧΪ�ⶨCu��CuO�����Ʒ��CuO�ĺ������ס��ҡ���������λͬѧ���ȳ�ȡ20g��Ʒ���ձ��У��ڷֱ����20g��40g��60g��80g��������������ͬ��ϡ���ᣮ��ַ�Ӧ���й���ʣ�࣮�����ˡ�����������ʣ�������������ͼ��ʾ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com