
 =35%���ʴ�Ϊ��35%��
=35%���ʴ�Ϊ��35%��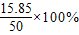 =31.7%��
=31.7%��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011�������ʡ���������п���ѧģ���Ծ��������������棩 ���ͣ�ѡ����
| ѡ�� | �����ֵ����� | ���ֵķ����ٺͷ����� |
| A | ľ̿�ۡ�����ͭ | �ٹ۲���ɫ��ͨ��CO������ |
| B | �� �״� | �ٵ�ȼ �ڹ۲���ɫ |
| C | ����������Һ��������Һ | ����֬ �ڷ�̪��Һ |
| D | ������̼������ | ����ˮ�Т�ȼ�ŵ�ľ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2007���п���ѧһģ�Ծ��������棩 ���ͣ�ѡ����
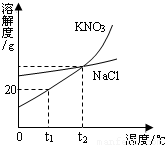
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008�꽭��ʡ�������ٴ������п���ѧ��ģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008�꽭��ʡ�������ٴ������п���ѧ��ģ�Ծ��������棩 ���ͣ�ѡ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com