��2012?��������ģ��������������˵�Ŭ�����й�����ʵ���ˡ����족���룬ʵ�֡����족������Ҫ�˷�������������ѣ����С�̫�ղտ�������̫�շ����������е����
��1��̫�շ���Ҫ��������ܣ�����Ҫ�ø�ǿ�ȵĵ��ڵȲ��ϣ���Ҫ���Զ��ֽ����ͽ�ճ�����Ƴɣ�������Ļ�ѧ֪ʶ�жϣ�����
��
��
����ǡ�������������Ȼ��ά��
��2��̫�շ�����Ҫ���ȡ�ɢ���⣬��Ҫ��ͨѶϵͳ������ϵͳ����������ϵͳ�ͷ�������ϵͳ�����з������������������裺
��һ�����÷�������һ��װ��ľ̿�ĺ��ӳ�ȥ��������һ����������ľ̿��
����
����
�ԣ�
�ʶ���������������ﮣ�LiOH�������ռ���ȥ������̼��������﮺��������ƶ��Ǽ�������ƵĻ�ѧ���ʣ���д���÷�Ӧ�Ļ�ѧ����ʽ
2LiOH+CO2�TLi2CO3+H2O
2LiOH+CO2�TLi2CO3+H2O
��
��3����̫�ղ��NiFe
2O
4�����ڴٽ��Ա������CO
2ת��ΪO
2������������������ѧ���ʱ��ֲ��䣬����ΪNiFe
2O
4����һ��Ӧ����
����
����
��
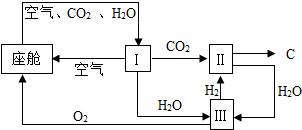
��4����������������ڿ������¹�����ͼ��ʾ��
��װ�â���CO
2��H
2�ķ�Ӧװ�ã��÷�Ӧ�Ļ�ѧ����ʽΪ
CO2+2H2=C+2H2O
CO2+2H2=C+2H2O
���ɲ�д����Ӧ��������
��װ�â�����Ӧ�Ļ�ѧ����ʽΪ
��
�۴�װ�â�ɿ�����O
2����Դ��CO
2��H
2O��������896g O
2������1012g CO
2����ͬʱ����H
2O
180
180
g��

 ��2012?��������ģ��������������˵�Ŭ�����й�����ʵ���ˡ����족���룬ʵ�֡����족������Ҫ�˷�������������ѣ����С�̫�ղտ�������̫�շ����������е����
��2012?��������ģ��������������˵�Ŭ�����й�����ʵ���ˡ����족���룬ʵ�֡����족������Ҫ�˷�������������ѣ����С�̫�ղտ�������̫�շ����������е����


 ��2012?��������ģ��ͼ������A��B��C���ܽ�����ߣ��Ը���ͼ��ش����⣮
��2012?��������ģ��ͼ������A��B��C���ܽ�����ߣ��Ը���ͼ��ش����⣮