
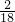 ��100%=0.24g��̼Ԫ�ص�����=5.28g��
��100%=0.24g��̼Ԫ�ص�����=5.28g��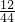 ��100%=1.44g������1.44g+0.24g=1.68g��2.16g�����������غ㶨�ɿ���֪���������к�����Ԫ�أ�������Ϊ��2.16g-1.68g=0.48g��
��100%=1.44g������1.44g+0.24g=1.68g��2.16g�����������غ㶨�ɿ���֪���������к�����Ԫ�أ�������Ϊ��2.16g-1.68g=0.48g�� 12CO2+5H2O
12CO2+5H2O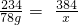


 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʵ������ȼƷ��������һƿʧȥ��ǩ���Լ���ij��ѧ��ȤС��ͬѧΪ̽������ɣ�����������ʵ�顣
��1����ͬѧȡ�����������������������г��ȼ�գ���������������ˮ�Ͷ�����̼���ɴ˿ɵó��Ľ�����
��ѡ����ţ���
| A����������ֻ����̼����Ԫ�� |
| B����������ֻ����̼����Ԫ�� |
| C����������һ������̼����Ԫ�أ����ܺ�����Ԫ�� |
| D����������̼���⡢������Ԫ����� |
��2����ͬѧȡ3.6g�������������������г��ȼ�գ����ⶨ����5.4gˮ��11g������̼��5.4gˮ�к���Ԫ�� g��11g������̼�к�̼Ԫ�� g����������� ��Ԫ�أ�ѡ����С�����������
��3����ͬѧ������ͬѧ��ʵ�����ݺͷ����������һ���������������̼ԭ�Ӻ���ԭ�ӵĸ�����Ϊ ������������ȣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2009�꽭��ʡ�������������п���ѧһģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com