��1���뽫��������ʵ�����Ŀ����ȷѡ����ڶ�Ӧ�ĺ����ϣ�
A����ֹҩƷ��Ⱦ B����ֹ�Թ����� C����ֹʵ����� D����ֹҺ�彦��
�ټ����Թ�ʱ���Ⱦ��ȼ��ȣ����м��ȣ�
�ڵι�ʹ�ú�ʱ��ϴ�������Թܼ��ϣ�
�۹���ʱ�����Һ�ز�������������©���У�
����ȡҺ��ʱ�������밼Һ����ʹ�����ƽ�ӣ�
��2����ѧʵ����������ڻ�ѧѧϰ���о��о�����Ҫ���ã�����a�Թܡ�bȼ�ճס�c�ƾ��ơ�d����ƿ��eҩ�ס�f��ͷ�ιܡ�g��Ͳ��10mL��50mL��100mL������������Ϊ����ʵ�����ѡ��������
�ٿ���ֱ�Ӽ��ȵ������� �����������գ�
����ȡ8.5mLˮ �����������գ�
��ijͬѧ����98%��Ũ���ᣨ�ܶ�Ϊ1.84g/cm3��������10%��ϡ���ᣮ
������Һ�����У���Ҫ��ȡ80mL��Ũ���ᣬѡ�� mL����Ͳ������ʱ����ͬѧ������Ͳ�Ŀ̶��ߣ�������������������Ũ�������� ���ƫ��ƫС������Ӱ�족����
���𰸡���������1���ٸ��Թܼ���ʱ���Թ����ѵĿ���ԭ���кࣺܶ�ټ���ǰû�и��Թ�Ԥ�ȣ����Ȳ����ȣ����Թ������ˮ������ʱû���ɣ����ԹܽӴ�����о��
��ʹ�ý�ͷ�ιܺ�������ϴ����ֹ��Ⱦ���ҩƷ��
�۹���ʱ�����Һ�ز�������������©�������������������ã���ֹҺ�彦����
����ȡҺ��ʱ������û��Һ��İ�Һ����ʹ�����ˮƽ����ȡҺ��ʱ���ٸ��ӣ���ȡ��Һ��ʵ�����ƫ�����ӣ���ȡ��Һ��ʵ�����ƫС��
��2���ٿ�ֱ���ھƾ����ϼ��ȵ��������Թܡ�������������ȼ�ճף�
�ڸ��ݼ�����������
�۸�����ȡ80mL��Ũ����ȷ�����õ����������Ӷ���ʱ��ʵ��ֵƫ��������ȡŨ����������ƫС��
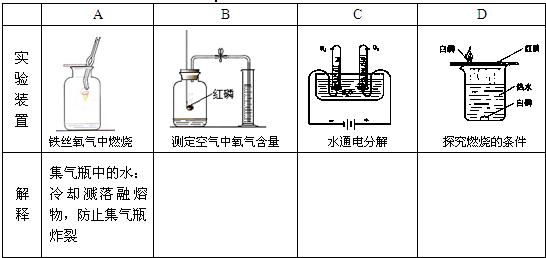
����⣺��1���ټ����Թ�ʱ���Ⱦ��ȼ��ȣ����м��ȣ���ֹ�Թ����ѣ�
�ڵι�ʹ�ú�ʱ��ϴ�������Թܼ��ϣ���ֹҩƷ��Ⱦ��
�۹���ʱ�����Һ�ز�������������©���У���ֹҺ�彦����
����ȡҺ��ʱ�������밼Һ����ʹ�����ƽ�ӣ���ֹʵ����
�ʴ�Ϊ����B����A����D����C��
��2���ٿ�ֱ���ھƾ����ϼ��ȵ�������a�Թܺ�bȼ�ճף�
����ȡ8.5mLˮ����10mL����Ͳ�ͽ�ͷ�ιܣ�
����ȡ80mL��Ũ����ʹ��100mL ����Ͳ����Σ����軹�轺ͷ�ιܶ��ݣ����Ӷ���ʱ��ʵ��ֵƫ��������ȡŨ����������ƫС��
�ʴ�Ϊ����a��b�� ��g��f�� ��100�� ƫС��
������������Ͳ����ͷ�ιܵ�ʹ�÷������˽���Թ��ڹ����Һ����ȵķ��������չ��˵�ԭ������������Ӧ�ã�������Ҫ���鳣�����������ã����Ƽ���ͬѧ��Ҫϸ�IJſ����Դ��⣮



 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
 24��ʵ��װ�ü�ʵ������ǻ�ѧʵ��Ļ�������
24��ʵ��װ�ü�ʵ������ǻ�ѧʵ��Ļ�������