
ˮ������������Ȼ��Դ��������ѧ��ѧ֪ʶ�ش�
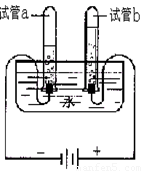
��1��С��������ͼ��ʾ��װ��̽��ˮ����ɣ�д���÷�Ӧ�Ļ�ѧʽ����ʽ ��ͨ��һ��ʱ����Թ�a�ռ����������� �������븺������������������ ��Ϊ�� ����Ҫ��ˮ�м���ϡ���������������Һ��
��2�������϶�һ�š� ����̫�յĻ����������Ϊ����ʹ�õ�ȼ����Һ�⡢��ȼ����Һ������Һ��ȼ�յĻ�ѧʽ����ʽΪ ��
��3��Ϊ��̽����ˮ�ľ��������̣�ijʵ��С��ӻ��Ǻ���ȡ��ˮ�����۲쵽ˮ�����ǣ��ʻ�ɫ������ζ���й���С�������ֶ�ˮ���������´������裺
�ټ������̿����ˮ�е���ɫ����ζ��
����ˮ���м������������ܽ⣬����һ��ʱ����� ����������ƣ�����ȥ��ˮ���еĹ���С��������������ò���������Һ�Ծɻ��ǣ������һ�����ԭ������� ��
��4������ˮ���ö�����������ɱ�����������ȵĻ�ѧʽΪ ��������Ԫ�صĻ��ϼ�Ϊ ��
��5����ˮ�к��н϶�ĸơ�þ���ӣ�Ϊ����Ӳ�ȣ��ճ�������ʹ��Ӳˮ����������鷳����ͥ�����г���������ˮӲ�ȵķ����� ��
��1��H2O H2 +O2
����
1:2 ��ǿˮ�ĵ�����
H2 +O2
����
1:2 ��ǿˮ�ĵ�����
��2��H2+O2 H2O
H2O
��3������ ��ֽ���𣨻����������𰸣�
��4��ClO2 +4
��5�����
��������
�����������1�����ˮʱ���������������������������������������������Ϊ1:2������ˮ�����磬���ˮʱ����Ҫ��ˮ�м���ϡ���������������Һ����Ϊ����ǿˮ�ĵ����ԡ���2���ԡ���3��ˮ�ľ��������ǣ����������ˡ���������������������˲���������Һ�Ծɻ��ǣ������һ�����ԭ���������ֽ�����Һ�������ֽ��Ե�ȡ���4�����ݻ�������Ԫ�ص��������ϼ۵Ĵ�����Ϊ��� X+��-2��*2=0 �� X= +4����5�� ����ˮӲ�ȵķ�������л�����ͥ�����г���������ˮӲ�ȵķ�������С�
���㣺ˮ�ĵ��ʵ�飬ˮ�ľ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
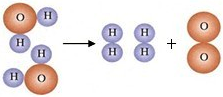
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
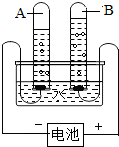 ˮ������������Ȼ��Դ����ͼ�ǵ��ˮ��ʵ��װ�ã��ش��������⣺
ˮ������������Ȼ��Դ����ͼ�ǵ��ˮ��ʵ��װ�ã��ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2010?�Թ���ˮ������������Ȼ��Դ����ͼ�ǵ��ˮ��ʵ��װ�ã��ش��������⣺
��2010?�Թ���ˮ������������Ȼ��Դ����ͼ�ǵ��ˮ��ʵ��װ�ã��ش��������⣺
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ˮ������������Ȼ��Դ��С��������ͼ��ʾ��װ��̽��ˮ����ɣ�ͨ��һ��ʱ����Թ�A���Թ�B���ռ������������֮��ԼΪ
ˮ������������Ȼ��Դ��С��������ͼ��ʾ��װ��̽��ˮ����ɣ�ͨ��һ��ʱ����Թ�A���Թ�B���ռ������������֮��ԼΪ
| ||
| ||
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com