���⻯ѧ��ȤС���ͬѧ����ij�������ķϼ�Һ����Ҫ�ɷ�ΪNa
2CO
3������������NaCl���������ʲ��ƣ���ʯ����Ϊԭ���Ʊ��ռ�������õ��ռ�ֲ�Ʒ�ijɷֽ��з����Ͳⶨ��
��һ���ֲ�Ʒ�Ʊ���
��1�����ϼ�Һ��������Ũ�����γɽ�Ũ����Һ����ȴ����ʯ�����ϣ�������Ӧ�Ļ�ѧ����ʽΪ��
��2������Ӧ��Ļ������ˣ��õ�����Һ���������ᾧ���Ƶôֲ�Ʒ��
�������ֲ�Ʒ�ɷַ�����
��1��ȡ�����ֲ�Ʒ����ˮ���μ�Ba��NO
3��
2��Һ���ְ�ɫ���ǣ�������Ӧ�Ļ�ѧ����ʽΪ���ôֲ�Ʒ��һ�������У�
�����ǣ�
��2����С��ͬѧͨ���Դֲ�Ʒ�ɷֵ�ʵ�������ȷ���ôֲ�Ʒ�к����������ʣ�
�������ֲ�Ʒ�����ⶨ��
[Na
2CO
3�����IJⶨ]
��1������ȤС���ͬѧ�������ͼ��ʾ��ʵ��װ�ã�ȡ10.0g�ֲ�Ʒ������ʵ��

˵������ʯ����CaO��NaOH�Ĺ������Eװ���еı���NaHCO
3��Һ��Ϊ�˳�ȥ������̼�����е��Ȼ��⣬�����ķ�ӦΪ NaHCO
3ʮHCl=NaClʮCO
2��ʮH
2O��
��2����������
�����Ӻ�װ�ã���������ԣ��ڴ��ɼ�C����A������ͨ��һ��ʱ��������۳���G���������ܹرյ��ɼ�C�������μ�Ũ������������ֱ��D��������ð�����ݴ��ɼ�C���ٴλ���ͨ��һ��ʱ����������ٴγ���G����������ǰ������������Ϊ0.48g��
��3������̽��
F�е��Լ�ӦΪ��Bװ�õ������ǣ�
Hװ�õ������ǣ�
��û��Hװ�ã���ⶨ��Na
2CO
3�����������ᣨ�ƫ����ƫС���������䡱����
��ʵ��10.0g�ֲ�Ʒֻ�ܲ���0.44g CO
2��������ϸ��������ʵ�飬����ʵ��ֵ0.48g����ȷֵ0.44gƫ���ԭ�����������ȷ����
��4�����ݼ���
������ȷֵ0.44g����ôֲ�Ʒ��Na
2CO
3����������Ϊ%��
[NaOH�����IJⶨ]
��С��ͬѧ����ȡ10.0g�ֲ�Ʒ����μ���20%��������ǡ����ȫ��Ӧʱ���������������Ϊ36.5g���ų�CO
20.44g�������Ƕ�����̼������ܽ⣩����ԭ�ֲ�Ʒ��NaOH��������������д��������̣�


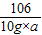 =
=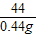 ��a=10.6%
��a=10.6%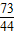 =
=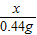 ��x=0.73g
��x=0.73g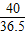 =
=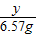



 ijУ��ѧ������ȤС���ͬѧ���о���ѧϰ����չʾ��һ������ͼ��ʾʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ��μӷ�Ӧ�����������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ��H2C2O4
ijУ��ѧ������ȤС���ͬѧ���о���ѧϰ����չʾ��һ������ͼ��ʾʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ��μӷ�Ӧ�����������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ��H2C2O4
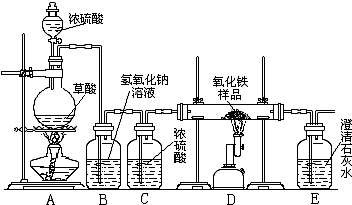
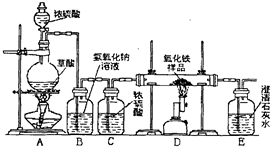 ijУ��ѧ������ȤС���ͬѧ���о���ѧϰ��չʾ��һ����ͼ��ʾ��ʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ���Ӧ�����������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ��
ijУ��ѧ������ȤС���ͬѧ���о���ѧϰ��չʾ��һ����ͼ��ʾ��ʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ���Ӧ�����������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ�� CO��+CO2��+H2O
CO��+CO2��+H2O