

| ��1�� | ��3�� | ��4�� | |
| ���������������g�� | 25 | 25 | 25 |
| ʣ������������g�� | 45 | a | 30 |
���� ��1�����ݷ�Ӧ��ʣ�������������μӷ�Ӧ��̼���������Ȼ�����̼��Ƶ�����������
���ݱ��е����ݽ��з�����
��2�����ݻ�ѧ����ʽ�����е����ݼ���μӷ�Ӧ���Ȼ��⣬Ȼ�����ϡ�������������������
��3���������е����ݼ��㷴Ӧ��������������Һ������Ȼ�������������������
��� �⣺��1����Ʒ�Ʋ���ҩ����CaCO3������������$\frac{50g-30g}{50g}$��100%=40%��
�ɵ�1�κ͵�4�����ݿ�֪25gϡ���������5g̼��ƣ���ڶ��μ���25gϡ�����ʣ�����Ϊ40g�������μ���25gϡ�����ʣ�����Ϊ35g����a����ֵΪ35��
��2����μӷ�Ӧ��������HCl������Ϊx���μӷ�Ӧ��CaCO3������Ϊy�������Ȼ��Ƶ�����Ϊz
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 111 44
y x z 8.8g
x=14.6g
y=20g
z=22.2g
������HCl������������$\frac{14.6g}{100g}$��100%=14.6%��
��3��������Һ������Ϊ��20g+100g-8.8g=111.2g��
������Һ��������������Ϊ��$\frac{22.2g}{111.2g}$��100%=20.0%��
��������HCl����������Ϊ14.6%��������Һ��������������Ϊ20.0%��
�ʴ�Ϊ����1��40%��35��
��2��������HCl������������14.6%��
��3��������Һ��������������Ϊ��20.0%��
���� ������Ҫ����ѧ�����û�ѧ����ʽ���м����������������ѧ���������ݺ۲�ͼ�������������������


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
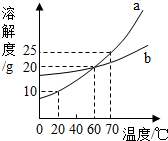 ��ͼΪa��b�����ʵ��ܽ�����ߣ����ͼ�ش�
��ͼΪa��b�����ʵ��ܽ�����ߣ����ͼ�ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��HCl��NaOH��Na2CO3��Ca��OH��2��Һ��ȡ�����е����ֻ�ϣ�
��HCl��NaOH��Na2CO3��Ca��OH��2��Һ��ȡ�����е����ֻ�ϣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
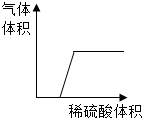
 �Ȼ��ơ�����ء������ӵ��ܽ��������ͼ��ʾ����ͼ�ش�
�Ȼ��ơ�����ء������ӵ��ܽ��������ͼ��ʾ����ͼ�ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���ʣ�������Ϊ���ʣ� | �����Լ����������� |
| A | FeSO4��CuSO4�� | ����������ˮ���������������ۣ����ˣ������ᾧ |
| B | NaOH��Һ��Na2CO3�� | ������������������Һ������ |
| C | ͭ�ۣ���������ĩ�� | ��������ϡ���ᣬ���ˣ�ϴ�ӣ����� |
| D | N2��O2�� | ������ͨ�����ȵ�̿�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
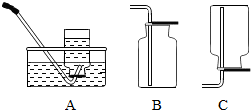 ���㽭���ߡ�ˮ����𣬿��������ⳡ�꣬ȴ��һ����ֵ��г䵱�ˡ��͡��Ľ�ɫ��������30�ҳ�һ·�������֡����ϴ�������ʯ��13Сʱ!��һ�ж�����Ϊһ������20��ֵ�ʯ�Ĵ�������ݽ��ܣ���ʯ����ѧ����̼���ƣ�CaC2��������ȼ����һ���л��ϳɻ�ѧ��ҵ�Ļ���ԭ�ϣ���ѧ���ʷdz����ã���ˮ���ܼ��ҷֽ������Ȳ��C2H2��������������ƣ��ͷų��������ȣ���Ȳ���岻������ˮ���ܶȱȿ���С����ش�
���㽭���ߡ�ˮ����𣬿��������ⳡ�꣬ȴ��һ����ֵ��г䵱�ˡ��͡��Ľ�ɫ��������30�ҳ�һ·�������֡����ϴ�������ʯ��13Сʱ!��һ�ж�����Ϊһ������20��ֵ�ʯ�Ĵ�������ݽ��ܣ���ʯ����ѧ����̼���ƣ�CaC2��������ȼ����һ���л��ϳɻ�ѧ��ҵ�Ļ���ԭ�ϣ���ѧ���ʷdz����ã���ˮ���ܼ��ҷֽ������Ȳ��C2H2��������������ƣ��ͷų��������ȣ���Ȳ���岻������ˮ���ܶȱȿ���С����ش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ��ʯ������ȼ�� | B�� | �ƹ�ʹ��̫������ˮ�� | ||
| C�� | ������ú�������� | D�� | ��ֹ��˽�ҳ����� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com