
ĪŖ²ā¶ØijŹÆ»ŅŹÆѳʷ֊Ģ¼ĖįøʵÄŗ¬Įæ£¬Č”ŃłĘ·10gÓŚÉÕ±ÖŠ£¬ŌŁĻņĘäÖŠ¼ÓČėĻ”ŃĪĖį50g£¬Ē”ŗĆĶźČ«·“Ó¦£ØŌÓÖŹ²»·“Ó¦£©£¬·“Ó¦ŗóÉÕ±ÖŠĪļÖŹ×ÜÖŹĮæĪŖ56.7g”£
£ØCaCO3£«2HCl£½CaCl2£«CO2”ü£«H2O£©
£Ø1£©Éś³ÉCO2µÄÖŹĮæĪŖ_______ g”£
£Ø2£©ĒóŹÆ»ŅŹÆѳʷ֊Ģ¼ĖįøʵÄÖŹĮæ·ÖŹż”£
”¾½āĪö”æ£Ø1£©øł¾ŻÖŹĮæŹŲŗć¶ØĀÉæÉÖŖÖŹĮæµÄ¼õÉŁĮæ¾ĶŹĒÉś³É¶žŃõ»ÆĢ¼µÄÖŹĮ棻£Ø2£©øł¾Ż¶žŃõ»ÆĢ¼µÄÖŹĮæĖć³öĢ¼ĖįøʵÄÖŹĮ棬ŌŁ³żŅŌѳʷ֏Į漓æÉ£®
£Ø1£©3.3 £Ø2·Ö£©
£Ø2£©¼ŁÉč”¢“š”¢µ„Ī» ------£Ø1·Ö£©
½ā£ŗÉčŹÆ»ŅŹÆѳʷ֊ŗ¬Ģ¼ĖįøʵÄÖŹĮæĪŖX”£
CaCO3 + 2HCl = CaCl2 + CO2ӟ+ H2O
100 44
X 3.3g ---------£Ø1·Ö£©
100£Æ44=X£Æ3.3g -----£Ø1·Ö£©
½āµĆ£ŗ X=7.5g -----£Ø1·Ö£©
ѳʷ֊Ģ¼ĖįøʵÄÖŹĮæ·ÖŹżĪŖ£ŗ
![]() =75% -------£Ø2·Ö£©
=75% -------£Ø2·Ö£©
“š£ŗŹÆ»ŅŹÆѳʷ֊ŗ¬Ģ¼ĖįøʵÄÖŹĮæ·ÖŹżĪŖ75%”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
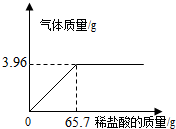 æĪĢāŠ”×éĪŖ²ā¶ØijŹÆ»ŅŹÆѳʷ֊CaCO3µÄŗ¬Įæ£¬Č”l0gŹÆ»ŅŹÆѳʷ·ÅŌŚÉÕ±ÖŠ£¬Č»ŗóĻņĘä֊עČėŅ»¶ØĮæÄ³ÖŹĮæ·ÖŹżµÄĻ”ŃĪĖį£¬Ź¹Ö®Óėѳʷ³ä·Ö·“Ó¦£ØŌÓÖŹ²»²ĪÓė·“Ó¦£©£®Ėę·“Ó¦½ųŠŠ£¬×¢ČėĻ”ŃĪĖįµÄÖŹĮæÓė·“Ó¦µĆµ½ĘųĢåµÄÖŹĮæ³ŹČēĶ¼ĖłŹ¾¹ŲĻµ£®ĒėĶź³ÉĻĀĮŠ¼ĘĖćÄŚČŻ£ŗ
æĪĢāŠ”×éĪŖ²ā¶ØijŹÆ»ŅŹÆѳʷ֊CaCO3µÄŗ¬Įæ£¬Č”l0gŹÆ»ŅŹÆѳʷ·ÅŌŚÉÕ±ÖŠ£¬Č»ŗóĻņĘä֊עČėŅ»¶ØĮæÄ³ÖŹĮæ·ÖŹżµÄĻ”ŃĪĖį£¬Ź¹Ö®Óėѳʷ³ä·Ö·“Ó¦£ØŌÓÖŹ²»²ĪÓė·“Ó¦£©£®Ėę·“Ó¦½ųŠŠ£¬×¢ČėĻ”ŃĪĖįµÄÖŹĮæÓė·“Ó¦µĆµ½ĘųĢåµÄÖŹĮæ³ŹČēĶ¼ĖłŹ¾¹ŲĻµ£®ĒėĶź³ÉĻĀĮŠ¼ĘĖćÄŚČŻ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗ³õÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com